Abstract
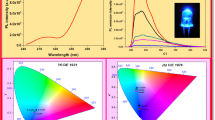
Here the green-emitting highly luminescent Er3+ doped, Er3+-Li+ co-doped, Er3+-Na+ co-doped CaAl4O7 is synthesized by Pechini method at 1000°C. Photoluminescence (PL) of CaAl4O7: Er3+ studies have been compared with Li+ co-doped CaAl4O7: Er3+ and Na+ co-doped CaAl4O7: Er3+. Na+ co-doped CaAl4O7:Er3+ shows increases in luminescence intensity compared to Li+ co-doped CaAl4O7: Er3+ and Er3+ doped CaAl4O7. The results suggest that CaAl4O7:Er3+ phosphor can be used as efficient green-emitting phosphor in white LED. The resultant phosphor emits green color peaking at 549 nm upon 378 nm excitation. Powder X-ray diffraction (PXRD) and photoluminescence (PL) techniques have been studied to characterize the synthesized microparticles. Further, this phosphor has good thermal stability that implies its potential to act as green phosphor in white light-emitting diodes. The effect of activator (Er3+), Na+ co-doped CaAl4O7:Er3+, and Li+ co-doped CaAl4O7:Er3+ phosphors luminescence spectra as well as photoluminescence life time studies were studied in detail. The results show that as the concentration of Er3+ in CaAl4O7 increases, the symmetry around the Er3+ ion decreases due to the creation of lattice defects in the crystal. Addition of Na+ and Li+ ions in CaAl4O7: Er3+leads to a small distortion in the local symmetry of Er3+ ions, thereby significantly enhancing its luminescence property. Analysis of photoluminescence life time studies of the prepared samples shows a smaller concentration quenching of Er3+ luminescence in charge compensated Na+ and Li+ CaAl4O7 phosphor.


















Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the first and corresponding author on reasonable request.
Code availability
No code usage involved in this paper.
References
A. Shalav, B.S. Richards, M.A. Green, Sol. Energy Mater. Sol. Cells. 91, 829 (2007)
M. Miritello, P. Cardile, R.L. Savio, F. Priolo, Opt. Express 19, 2076 (2011)
L. Wang, N. Liao, H. Zeng, L. Shi, H. Jia, N. Wang, S. Guo, D. Jin, Thin Solid Films 20, 174 (2011)
Xu. Li, Z. Yang, Li. Guan, J. Guo, Y. Wang, Q. Guo, J. Alloys Compds. 478, 684 (2009)
M. Ayvacikli, A. Ege, S. Yerci, N. Can, J. Lumin. 131, 2432 (2011)
V. Singh, R. P. S Chakradhar, I. Ledoux Rak, L. Badie, F. Pelle, S. Ivanova, J. Lumin. 129, 1375 (2009)
J. Lin, Yu. Min, C. Lin, X. Liu, J. Phys. Chem. C 111, 5835 (2007)
A. Suresh Kumar, R. Arun Kumar, R. Balasundaraprabhu, K. Senthil, S. Ramesh Kumar, Spectrochim. Acta part A Mol. Biomol. Spectrosc. 134, 283–287 (2015)
A. Suresh Kumar, R. Arun Kumar, R. Ranjan Bhattacharjee, J. Lumin. 182, 130 (2017)
W. Liu, G.C. Farrington, F. Chaput, B. Dumn, J. Electrochem. Soc. 143, 879 (1996)
K. Sun, L.H. Oh, Ind. Eng. Chem. 35, 37 (1996)
K. Mondal, P. Kumari, J. Manam, Curr. Appl. Phys. 16, 7 (2016)
M. Puchalska, A. Watras, J. Solid State Chem. 238, 259 (2016)
S. Saha, S. Das, U.K. Ghorai, N. Mazumder, B.K. Gupta, K.K. Chattopadhyay, Dalton Trans. 42, 12965 (2013)
J.H. Chung, J.H. Ryu, J.W. Eun, J.H. Lee, S.Y. Lee, T.H. Heo, K.B. Shim, Mater. Chem. Phys. 134, 695 (2012)
Y. Bai, Y. Wang, G. Peng, K. Yang, X. Zhang, Y. Song, J. Alloys Compds. 478, 676 (2009)
G. Chen, H. Liu, H. Liang, G. Somesfalean, Z. Zhang, J. Phys. Chem. C 112, 12030 (2008)
Y.C. Li, Y.H. Chang, Y.F. Lin, Y.J. Lin, Y.S. Chang, Appl. Phys. Lett. 89, 81110 (2006)
S.A. Yan, J.W. Wang, Y.S. Chang, W.S. Hwang, Y.H. Chang, Opt. Mater. 34, 147 (2011)
R.F. Qiang, S. Xiag, J.W. Ding, W. Yuan, C. Zhu, J. Lumin. 129, 826 (2009)
T. Li, C. Guo, Y. Wu, L. Li, J.H. Jeong, J. Alloys Compds. 540, 107 (2012)
B. Liu, M. Gu, X. Liu, C. Ni, D. Wang, L. Xiao, R. Zhang, J. Alloys Compds. 440, 341 (2007)
Q. Dou, Y. Zhang, Langmuir 27, 13236 (2011)
A.K. Sing, S.K. Singh, S.B. Rai, R. Soc, Chem. Adv. 4, 27039 (2014)
M. Puchalska, E. Zych, J. Lumin. 132, 826 (2012)
M. Inokuti, F. Hirayama, J. Chem. Phys. 43, 1978 (1965)
Acknowledgements
The authors (A. Suresh Kumar) acknowledge University Grants Commission––Hyderabad (UGC)—for the financial support in the form of minor research project [No: MRP—4982/14 (SERO/UGC)].
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dr. A. Suresh Kumar and Dr. R. Arun Kumar. The first draft of the manuscript was written by the corresponding authors Dr. A. Suresh Kumar and the Dr. R. Arun Kumar commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A.S., Kumar, R.A. & Vijayakumar, R. Synthesis and luminescence properties of Na+, Li+ compensated Er3+ doped CaAl4O7 phosphors. J Mater Sci: Mater Electron 33, 21297–21310 (2022). https://doi.org/10.1007/s10854-022-08916-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08916-0