Abstract
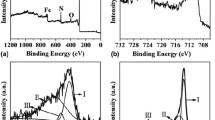
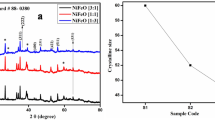
The electrocatalysis-based nickel oxide (NiO) films and doped with various amount of iron were synthesized on stainless steel (SS) substrate by electrodeposition. X-ray diffraction analysis (XRD) and filed effect scanning electron microscopy (FE-SEM) utilized to investigate structural and morphological properties. The X-ray diffraction analysis polycrystalline cubic structure has confirmed. Surface morphology study revealed the grains are regularly scattered over the total surface of the substrates after doping. The oxygen evolution reaction (OER) properties of doped films more efficient than that of pure thin films. The 4% Fe:NiO films exhibited an over potential of 320 mV at 10 mA cm−2 current density and a Tafel slope of 89.80 mV dec−1. The electrode shows the high durability over 5 h with small degradation.






Similar content being viewed by others
Data availability
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (htt://creative commons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
M.I. Jamesh, Recent progress on earth abundant hydrogen evolution reaction and oxygen evolution reaction bifunctional electrocatalyst for overall water splitting in alkaline media. J. Power Sources 333, 213–236 (2016)
M.I. Jamesh, A.S. Prakash, Advancement of technology towards developing Na-ion batteries. J. Power Sources 378, 268–300 (2018)
S.B. Lai, M.I. Jamesh, X.C. Wu et al., A promising energy storage system: rechargeable Ni–Zn battery. Rare Met. 36, 381–396 (2017)
S. Chu, A. Majumdar, Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012)
H. Wang, H. Dai, Strongly coupled inorganic-nano-carbon hybrid materials for energy storage. Chem. Soc. Rev. 42, 3088–3113 (2013)
T.R. Cook, D.K. Dogutan, S.Y. Reece, Y. Surendranath, T.S. Teets, D.G. Nocera, Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010)
Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier, H. Dai, Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011)
M.D. Symes, L. Cronin, Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nat. Chem. 5, 403–409 (2013)
M. Sathiya, K. Hemalatha, K. Ramesh, J.M. Tarascon, A.S. Prakash, Synthesis, structure and electrochemical properties of the layered sodium insertion cathode material: NaNi1/3Mn1/3Co1/3O2. Chem. Mater. 24, 1846–1853 (2012)
J.A. Turner, Sustainable hydrogen production. Science 305, 972–974 (2004)
N.S. Lewis, D.G. Nocera, Powering the planet: chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. Unit. States Am. 103, 15729–15735 (2006)
L.V. Wing-hei, K. Daniel, K. Hatice, P. Filip, P. Marie-Claire, R. Erwin, J. Gunnar, Dark photocatalysis: storage of solar energy in carbon nitride for time-delayed hydrogen generation. Angew. Chem. Int. Ed. 129, 525–529 (2017)
Z.L. Wang, D. Xu, J.J. Xu, X.B. Zhang, Oxygen electrocatalysts in metal-ion batteries: from aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 43, 7746–7786 (2014)
G.W. Crabtree, M.S. Dresselhaus, M.V. Buchanan, The hydrogen economy. Phys. Today 57, 39–44 (2004)
M.S. Dresselhaus, I.L. Thomas, Alternative energy technologies. Nature 414, 332–337 (2001)
C.L. Choi, J. Feng, Y. Li, J. Wu, A. Zak, R. Tenne, H. Dai, WS2 nanoflakes from nanotubes for electrocatalysis. Nano Res. 6, 921–928 (2013)
K. Zeng, D. Zhang, Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 36, 307–326 (2010)
M.G. Walter, E.L. Warren, J.R. McKone, S.W. Boettcher, Q. Mi, E.A. Santori, N.S. Lewis, Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010)
C.C.L. McCrory, S. Jung, I.M. Ferrer, S.M. Chatman, J.C. Peters, T.F. Jaramillo, Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015)
L. Ai, Z. Niu, J. Jiang, Mechanistic insight into oxygen evolution electrocatalysis of surface phosphate modified cobalt phosphide nonorod bundles and their superior performance for overall water splitting. Electrochim. Acta 242, 355–363 (2017)
P.T. Babar, B.S. Pawar, A.C. Lokhande, M.G. Gang, J.S. Jang, M.P. Suryawanshi, S.M. Pawar, J.K. Hyeok, Annealing temperature dependent catalytic water oxidation activity of iron oxyhydroxide thin films. J. Energy Chem. 26(4), 757–761 (2017)
S.M. Pawar, B.S. Pawar, B. Hou, J. Kim, A. Ahmed, H.S. Chavan, Y. Jo, S. Cho, A.I. Inamdar, J.L. Gunjakar, H. Kim, S. Cha, H. Im, Self-assembled two dimensional copper oxide nanosheet bundles as an efficient oxygen evolution reaction (OER) electrocatalyst for water splitting applications. J. Mater. Chem. A 5, 12747–12751 (2017)
P.T. Babar, A.C. Lokhande, B.S. Pawar, M.G. Gang, E. Jo, M.P. Changsik Go, S.M.P. Suryawanshi, J.H. Kim, Electrocatalytic performance evaluation of cobalt hydroxide and cobalt oxide thin films for oxygen evolution reaction. Appl. Surf. Sci. 427, 253–259 (2018)
H. Osgood, S.V. Devaguptapu, H. Xu, J. Cho, G. Wu, Transition metal (Fe Co, Ni and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 11(5), 601–625 (2016)
R.J. Deokate, S.H. Mujawar, H.S. Chavan, S.S. Mali, C.K. Hong, H. Im, A.I. Inamdar, Chalcogenide nanocomposite electrodes grown by chemical etching of Ni-foam as electrocatalyst for efficient oxygen evolution reaction. Int. J. Ener. Research 44(2), 1233–1243 (2020)
R.L. Doyle, I.J. Godwin, M.P. Brandon, M.E.G. Lyons, Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modifies electrods. Phys. Chem. Chem. Phys. 15, 13737–13783 (2013)
M. Cappadonia, J. Divisek, T. von der Heyden, U. Stimming, Oxygen evolution at nickel anodes in concentrated alkaline solution. Electrochim. Acta 39, 1559–1564 (1994)
B.S. Yeo, A.T. Bell, In situ raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. Phys. Chem. C 116, 8394–8400 (2012)
F. Foerster, A. Piguet, Physikalische Chemie 10, 714–721 (1904)
H.N. Seiger, R.C. Shair, Oxygen evolution from heavily doped nickel oxide electrodes. J. Electrochem. Soc. 108, C163–C163 (1961)
R. Subbaraman, D. Tripkovic, K.C. Chang, D. Strmcnik, A.P. Paulikas, P. Hirunsit, M. Chan, J. Greeley, V. Stamenkovic, N.M. Markovic, Trends in activity for the water electrolyser reactions on 3d M(Ni Co, Fe, Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012)
L. Han, S. Dong, E. Wang, Transition-metal (Co, Ni and Fe)- based electrocatalysts for the water oxidation reaction. Adv. Mater. 28, 9266–9291 (2016)
D.M. Jang, I.H. Kwak, E.L. Kwon, C.S. Jung, H.S. Im, K. Park, J. Park, Transition-metal doping of oxide nanocrystals for enhanced catalytic oxygen evolution. J. Phys. Chem. C 119(4), 1921–1927 (2015)
S. Han, S. Liu, S. Yin, L. Chen, Z. He, Electrodeposited Co-doped Fe3O4 thin films as efficient catalysts for the oxygen evolution reaction. Electrochim. Acta 210, 942–949 (2016)
A. Khan, M. Shkir, S.A. Ansari, S. Nazish Parveen, A.M. AlFaify, R.K. El-Toni, S.F.A. Gupta, One-pot flash combustion synthesis of Fe@NiO nanocomposites for supercapacitor applications. Ceram Int 47, 9024–9033 (2021)
Wu. Zhengcui, Z. Zou, J. Huang, F. Gao, Fe-doped NiO mesoporous nanosheets array for highly efficient overall water splitting. J. Catal. 358, 243–252 (2018)
M.W. Louie, A.T. Bell, An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 135, 12329–12337 (2013)
A.C. Pebley, E. Decolvenaere, T.M. Pollock, M.J. Gordon, Oxygen evolution on Fe-doped NiO electrocatalysts deposited via microplasma. Nanoscale 9, 15070–15082 (2017)
L. Trotochaud, S.L. Young, J.K. Ranney, S.W. Boettcher, Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014)
P. Wang, Z. Pu, Y. Li, L. Wu, Z. Tu, M. Jiang, Z. Kou, I.S. Amiinu, S. Mu, Iron-doped nickel phosphide nanosheet arrays: an efficient bifunctional electrocatalyst for water splitting. ACS Appl Mater. Interfaces 9, 26001–26007 (2017)
JCPDS card no. 47–1049
P. Scherrer, Bestimmung der grösse und der inneren struktur von kolloidteilchen mittels röntgenstrahlen. Nachr. Ges. Wiss. Göttingen 26, 98 (1918)
R.S. Kate, S.C. Bulakhe, R.J. Deokate, Co doping effect on structural and optical properties of nickel oxide (NiO) thin films via spray pyrolysis. Opt. Quant. Electron. 51(10), 1–19 (2019)
J. Yan, L. Kong, Y. Ji, J. White, Y. Li, J. Zhang, P. An, S. Liu, S.T. Lee, T. Ma, Single atom tungsten doped ultrathin α-Ni (OH)2 for enhanced electrocatalytic water oxidation. Nat. Commun. 10, 2149 (2019)
R.J. Deokate, H.S. Chavan, S.C. Bulakhe, S.B. Tanwade, S.H. Mujawar, S.S. Mali, C.K. Hong, H. Im, A.I. Inamdar, Electrodeposited bimetallic microporous MnCu oxide electrode as a highly stable electrocatalyst for oxygen evolution reaction. Short Commun, Int. J. Energy Res., 46(4), 5269–5279 (2022)
W. Gao, Z.M. Xia, F.X. Cao, J.C. Ho, Z. Jiang, Y.Q. Qu, Comprehensive understanding of the spatial configurations of CeO2 in NiO for the electrocatalytic oxygen evolution reaction: embedded or surface-loaded. Adv Funct. Mater 28, 1706056 (2018)
M. Dinca, Y. Surendranath, D.G. Nocera, Nickel-borate ̌ oxygen-evolving catalyst that functions under benign conditions. Proc. Natl. Acad. Sci. U. S. A. 107, 10337–10341 (2010)
K.-L. Yan, X. Shang, Z. Li, B. Dong, X. Li, W.-K. Gao, J.-Q. Chi, Y.-M. Chai, C.-G. Liu, Ternary mixed metal Fe-doped NiCo2O4 nanowires as efficient electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. 416, 371–378 (2017)
Acknowledgements
The authors would like acknowledge to Vidya Pratishtan’s, Arts, Science and Commerce College, Baramati, for support of Central Instrumentation Facility (CIF) by DST-FIST. Anekant Education Societies Tuljaram Chaturchand College, Baramati as research center.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
SCB: Writing-Original draft preparation, Methodology. RJ Deokate: Writing, reviewing and editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bulakhe, S.C., Deokate, R.J. Electrochemically prepared Fe: NiO thin film catalysis for oxygen evolution reaction. J Mater Sci: Mater Electron 33, 18180–18186 (2022). https://doi.org/10.1007/s10854-022-08674-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08674-z