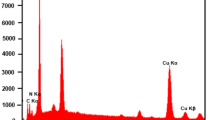
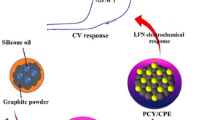
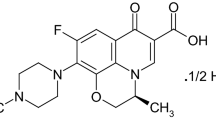
Abstract
Levofloxacin (LEV) is used in pharmaceuticals to treat bacterial infections, but is rarely metabolized by the human body, and hence, largely excreted. This leads to its accumulation in sewage, posing hazards to human health and the environment. Considering the drawbacks of existing methods to detect LEV, including a high cost, significant analysis time, and complex sample processing, the aim of this study was to devise an optimal detection method for LEV. A modified screen-printed electrode (SPE) using a Cu–metal–organic framework (MOF) derivative was proposed as an electrochemical sensor for LEV detection. They were characterized by transmission electron microscopy (TEM) and X-ray diffraction (XRD). The prepared Cu-MOF derivative offered a large specific surface area and highly dispersed active sites, which facilitated full contact with LEV. The electrochemical behavior of LEV was evaluated using cyclic voltammetry (CV) in the potential range of − 0.2 to 1 V. The linear response range was 0.1–100 μM, sensitivity was 1855 μA.mM−1.cm−2, and detection limit was 0.016 μM. The detection limit and sensitivity of differential pulse voltammetry (DPV) were 0.17 μM and 183 μA.mM−1.cm−2, respectively. The detection limit and sensitivity of chronoamperometry (CA) were 0.037 μM and 825 μA.mM−1.cm−2, respectively. The method exhibited significant selectivity and stability. The use of this electrochemical sensor containing the Cu-MOF derivative constitutes a simple and rapid method for determining LEV in medicine and the food industry.








Similar content being viewed by others
References
M. Rkik, M. Ben Brahirn, Y. Samet, Electrochemical determination of levofloxacin antibiotic in biological samples using boron doped diamond electrode. J. Electroanal. Chem. 794, 175–181 (2017). https://doi.org/10.1016/j.jelechem.2017.04.015
J.M. Wang, X. Lian, B. Yan, Eu3+-functionalized covalent organic framework hybrid material as a sensitive turn-on fluorescent switch for levofloxacin monitoring in serum and urine. Inorg. Chem. 58, 9956–9963 (2019). https://doi.org/10.1021/acs.inorgchem.9b01106
X.P. Tan, Q. Li, J.D. Yang, A simple fluorescence method detection levofloxacin in milk based on GSH-CdTe QDs. J. Mol. Struct. 1201, 4 (2020). https://doi.org/10.1016/j.molstruc.2019.127175
H. Zhad, R.Y. Lai, Iron(III)-mediated electrochemical detection of levofloxacin in complex biological samples. Electroanalysis 29, 2672–2677 (2017). https://doi.org/10.1002/elan.201700428
B. Liu et al., A sensitive electrochemical immunosensor based on PAMAM dendrimer-encapsulated au for detection of norfloxacin in animal-derived foods. Sensors 18, 11 (2018). https://doi.org/10.3390/s18061946
L.M. Feng et al., Voltammetric determination of ofloxacin by using a laser-modified carbon glassy electrode. Microchim. Acta 187, 8 (2020). https://doi.org/10.1007/s00604-019-4065-6
S. Pilehvar et al., A joint action of aptamers and gold nanoparticles chemically trapped on a glassy carbon support for the electrochemical sensing of ofloxacin. Sens. Actuator B-Chem. 240, 1024–1035 (2017). https://doi.org/10.1016/j.snb.2016.09.075
J.R. Ciorcero, G.N. Calaca, C.A. Pessoa, Carbon ceramic electrodes modified with mixed oxides SiO2/SnO2 for determination of levofloxacin. J. Solid State Electrochem. 22, 1403–1411 (2018). https://doi.org/10.1007/s10008-017-3794-x
A. Wong, T.A. Silva, F.C. Vicentini, O. Fatibello-Filho, Electrochemical sensor based on graphene oxide and ionic liquid for ofloxacin determination at nanomolar levels. Talanta 161, 333–341 (2016). https://doi.org/10.1016/j.talanta.2016.08.035
X.T. Zhou et al., Colorimetric determination of ofloxacin using unmodified aptamers and the aggregation of gold nanoparticles. Microchim. Acta 185, 9 (2018). https://doi.org/10.1007/s00604-018-2895-2
Y.M. Liu, J.T. Cao, W. Tian, Y.L. Zheng, Determination of levofloxacin and norfloxacin by capillary electrophoresis with electrochemiluminescence detection and applications in human urine. Electrophoresis 29, 3207–3212 (2008). https://doi.org/10.1002/elps.200800048
A.A. Salem, H.A. Mossa, B.N. Barsoum, Quantitative determinations of levofloxacin and rifampicin in pharmaceutical and urine samples using nuclear magnetic resonance spectroscopy. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 62, 466–472 (2005). https://doi.org/10.1016/j.saa.2005.01.016
M.H. Ghanbari et al., A nanocomposite consisting of reduced graphene oxide and electropolymerized -cyclodextrin for voltammetric sensing of levofloxacin. Microchim. Acta 186, 11 (2019). https://doi.org/10.1007/s00604-019-3530-6
C. Zhang et al., An electrochemical sensor based on plasma-treated zinc oxide nanoflowers for the simultaneous detection of dopamine and diclofenac sodium. Microchem J. 158, 8 (2020). https://doi.org/10.1016/j.microc.2020.105237
G.L. Zhang et al., Rapid synthesis of cypress-like CuO nanomaterials and CuO/MWCNTs composites for ultra-high sensitivity electrochemical sensing of nitrite. Microchem J. 159, 9 (2020). https://doi.org/10.1016/j.microc.2020.105439
Q.W. Bao et al., In situ detection of heavy metal ions in sewage with screen-printed electrode-based portable electrochemical sensors. Analyst 146, 5610–5618 (2021). https://doi.org/10.1039/d1an01012c
C.C. Kocak, Poly(taurine-glutathione)/carbon nanotube modified glassy carbon electrode as a new levofloxacin sensor. Electroanalysis 31, 1552–1561 (2019). https://doi.org/10.1002/elan.201900096
Y.F. Song et al., One-step rapid preparation of CuO nanosheets by high frequency induction heating and the application as excellent electrochemical sensor based on CuO/MWCNTs for the detection of glucose. Mater. Res. Express 6, 13 (2019). https://doi.org/10.1088/2053-1591/ab3ff2
Z.M. Jiang, Q. Liu, Y.R. Tang, M.X. Zhang, Electrochemical sensor based on a novel Pt-Au bimetallic nanoclusters decorated on reduced graphene oxide for sensitive detection of ofloxacin. Electroanalysis 29, 602–608 (2017). https://doi.org/10.1002/elan.201600408
W. Wang et al., Miniaturized device with a detachable three-electrode system and vibration motor for electrochemical analysis based on disposable electrodes. Sens. Actuator B-Chem. 297, 6 (2019). https://doi.org/10.1016/j.snb.2019.126719
H.D. Jin et al., Cu-based catalyst derived from nitrogen-containing metal organic frameworks for electroreduction of CO2. Acta Phys.-Chim. Sin. 37, 11 (2021). https://doi.org/10.3866/pku.Whxb202006017
X.L. Wang et al., Constructing NiCo/Fe3O4 Heteroparticles within MOF-74 for efficient oxygen evolution reactions. J. Am. Chem. Soc. 140, 15336–15341 (2018). https://doi.org/10.1021/jacs.8b08744
B.R. Kim, J.S. Oh, J. Kim, C.Y. Lee, Robust aerobic alcohol oxidation catalyst derived from metal-organic frameworks. Catal. Lett. 146, 734–743 (2016). https://doi.org/10.1007/s10562-016-1700-2
Y.Z. Chen, R. Zhang, L. Jiao, H.L. Jiang, Metal-organic framework-derived porous materials for catalysis. Coord. Chem. Rev. 362, 1–23 (2018). https://doi.org/10.1016/j.ccr.2018.02.008
M.H. Yap, K.L. Fow, G.Z. Chen, Synthesis and applications of MOF-derived porous nanostructures. Green Energy Environ. 2, 218–245 (2017). https://doi.org/10.1016/j.gee.2017.05.003
L. Jiao, J.Y.R. Seow, W.S. Skinner, Z.U. Wang, H.L. Jiang, Metal-organic frameworks: structures and functional applications. Mater. Today 27, 43–68 (2019). https://doi.org/10.1016/j.mattod.2018.10.038
Y.T. Liao, B.M. Matsagar, K.C.W. Wu, Metal-organic framework (MOF)-derived effective solid catalysts for valorization of lignocellulosic biomass. ACS Sustain. Chem. Eng. 6, 13628–13643 (2018). https://doi.org/10.1021/acssuschemeng.8b03683
C.S. Liu, J.J. Li, H. Pang, Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coord. Chem. Rev. 410, 39 (2020). https://doi.org/10.1016/j.ccr.2020.213222
H. Konnerth et al., Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 416, 24 (2020). https://doi.org/10.1016/j.ccr.2020.213319
C. Sarkar et al., Synthesis and characterization of mechanically strong carboxymethyl cellulose-gelatin-hydroxyapatite nanocomposite for load-bearing orthopedic application. J. Mater. Sci. 53, 230–246 (2018). https://doi.org/10.1007/s10853-017-1528-1
P.I. Scheurle et al., A highly crystalline anthracene-based MOF-74 series featuring electrical conductivity and luminescence. Nanoscale 11, 20949–20955 (2019). https://doi.org/10.1039/c9nr05431f
Z. Adamczyk et al., Colloid particle deposition on heterogeneous surfaces produced by polyelectrolyte adsorption. Colloids Surf. Physicochem. Eng. Aspects 343, 111–117 (2009). https://doi.org/10.1016/j.colsurfa.2009.01.037
B.M. Lee, C.U. Jeong, S.K. Hong, J.M. Yun, J.H. Choi, Eco-friendly fabrication of porous carbon monoliths from water-soluble carboxymethyl cellulose for supercapacitor applications. J. Ind. Eng. Chem. 82, 367–373 (2020). https://doi.org/10.1016/j.jiec.2019.10.036
L. Han, Y.F. Zhao, C. Chang, F. Li, A novel electrochemical sensor based on poly(p-aminobenzene sulfonic acid)-reduced graphene oxide composite film for the sensitive and selective detection of levofloxacin in human urine. J. Electroanal. Chem. 817, 141–148 (2018). https://doi.org/10.1016/j.jelechem.2018.04.008
M. Rostami et al., Facile synthesis and characterization of TiO2-graphene-ZnFe2-x Tb (x) O-4 ternary nano-hybrids. J. Mater. Sci. 52, 7008–7016 (2017). https://doi.org/10.1007/s10853-017-0934-8
Y.F. Jin et al., Fast cathodic reduction electrodeposition of a binder-free cobalt- doped Ni-MOF film for directly sensing of levofloxacin. J. Alloy. Compd. 851, 8 (2021). https://doi.org/10.1016/j.jallcom.2020.156823
V. Cesarino, I. Cesarino, F.C. Moraes, S.A.S. Machado, L.H. Mascaro, Carbon nanotubes modified with SnO2 rods for levofloxacin detection. J. Braz. Chem. Soc. 25, 502–508 (2014). https://doi.org/10.5935/0103-5053.20140017
J. Borowiec, K. Yan, C.C. Tin, J.D. Zhang, Synthesis of PDDA functionalized reduced graphene oxide decorated with gold nanoparticles and its electrochemical response toward levofloxacin. J. Electrochem. Soc. 162, H164–H169 (2015). https://doi.org/10.1149/2.0811503jes
F. Wang, L.H. Zhu, J.D. Zhang, Electrochemical sensor for levofloxacin based on molecularly imprinted polypyrrole-graphene-gold nanoparticles modified electrode. Sens. Actuator B-Chem. 192, 642–647 (2014). https://doi.org/10.1016/j.snb.2013.11.037
Acknowledgements
We acknowledge the support by Tianjin Natural Science Foundation (Grant No. 18JCZDJC99800), National Natural Science Foundation of China (Grant No. 51502203), Tianjin Distinguished Professor Foundation of Young Researcher, Tianjin Development Program for Innovation and Entrepreneurship, for research conducted at Tianjin University of Technology. The Ministry of Education (MOE2018-T2-2-095), for research conducted at the National.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. JZ, JL, PP, TL, ZY, PL, GL, HS and XZ: Material preparation, data collection and analysis were performed. JZ: The first draft of the manuscript was written and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Data availability
The authors declare that the other data supporting the findings of this study are available within the article and its supplementary information files.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, J., Liu, J., Pan, P. et al. Electrochemical determination of levofloxacin with a Cu–metal–organic framework derivative electrode. J Mater Sci: Mater Electron 33, 9941–9950 (2022). https://doi.org/10.1007/s10854-022-07985-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-07985-5