Abstract
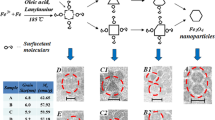
In various biomedical applications of iron oxide nanostructures, superparamagnetic nanoneedles offer benefits to access the interior of cells without damage. However, use of superparamagnetic nanoneedles should not be limited to biomedical applications. To explore and extend the field of application, this study is focused on detailed investigation of structural, magnetic, and field emission behavior of iron oxide. Iron oxide nanoneedles are prepared using template-free oleic acid-assisted sol–gel method by varying sols’ molarity in the range 0.2–2.0 mM (interval 0.2 mM). Formation of nanoneedles with fine tips and diameter of 20, 23, and 25 nm and length of 700 nm, 1.0 μm, and 1.2 μm at 0.2, 1.0, and 2.0 mM sols are confirmed using Scanning Electron Microscopy. While for rest of the molarity range studied diameter of nanoneedles increases to ~ 50 nm. At 0.2 mM sol magnetite (Fe3O4) phase is observed and vacancy ordered and disordered maghemite (γ-Fe2O3) phases are observed at 0.8–1.0 and 1.4–2.0 mM sols, respectively. Formation of these phases of iron oxide with variation in sols’ molarity is also confirmed using FTIR spectra and Raman spectroscopy. Iron oxide nanoneedles show soft magnetic behavior with high saturation magnetization of 73.2, 43.43, and 75.18 emu/g at 0.2, 1.0, and 2.0 mM sols, respectively. These magnetic nanoneedles have potential applications in cell probing. Furthermore, nanoneedles are also tested for their field emission properties. It is observed that nanoneedles, with disordered maghemite phase, synthesized using 2.0 mM sol have high field emission properties along with low turn-on field of 3.77 Vμm−1. Thus, this study reveals ordered and disordered phases of iron oxide using single route. Furthermore, these phases can be utilized for different applications, such as Fe3O4 (at 0.2 mM) phase for biomedical applications and γ-Fe2O3 phase as field emitters.











Similar content being viewed by others
Data availability
All the relevant data have been included in the manuscript.
References
R.R. Kumar, K.U. Kumar, D. Haranath, Synthesis, properties, and applications of transition metal oxide nanomaterials in multifunctional nanostructured metal oxides for energy harvesting and storage devices (CRC Press, Boca Raton, 2020), pp. 1–73
H.L. Fan, S.F. Zhou, G.S. Qi, Y.Z. Liu, Continuous preparation of Fe3O4 nanoparticles using impinging stream-rotating packed bed reactor and magnetic property thereof. J. Alloy. Compd. 662, 497–504 (2016)
P.I.P. Soares, F. Lochte, C.E. Echeverria, L.C.J. Pereira, J.T. Coutinho, I.M.M. Ferreira, C.M.M. Novo, J.P.M.R. Borges, Thermal and magnetic properties of iron oxide colloids: Influence of surfactants. Nanotechnology 26, 425704 (2015)
E. Fazio, M. Santoro, G. Lentini, D. Franco, S.P.P. Guglielmino, F. Neri, Iron oxide nanoparticles prepared by laser ablation: synthesis, structural properties and antimicrobial activity. Colloid Surf. A 490, 98–103 (2016)
D. Ling, N. Lee, T. Hyeon, Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Acc. Chem. Res. 48, 1276–1285 (2015)
S. Riaz, A. Akbar, S. Naseem, Controlled nanostructuring of multiphase core-shell iron oxide nanoparticles. IEEE Trans. Magn. 50, 2300204 (2014)
L.K. Joy, V. Sooraj, U.S. Sajeev, S.S. Nair, T.N. Narayanan, N. Sethulakshmi, P.M. Ajayan, M.R. Anantharaman, Large enhanced dielectric permittivity in polyaniline passivated core-shell nano magnetic iron oxide by plasma polymerization. Appl. Phys. Lett. 104, 121603 (2014)
M. Jiao, J. Zeng, L. Jing, C. Liu, M. Gao, Flow synthesis of biocompatible Fe3O4 nanoparticles: insight into the effects of residence time, fluid velocity, and tube reactor dimension on particle size distribution. Chem. Mater. 27, 1299–1305 (2015)
L.G. Moragas, S.M. Yu, N.M. Cremaes, A. Laromaine, A. Roig, Scale-up synthesis of iron oxide nanoparticles by microwave-assisted thermal decomposition. Chem. Eng. J. 281, 87–95 (2015)
G. Kandasamy, D. Maity, Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 496, 191–218 (2015)
X. Shi, B. Chu, F. Wang, X. Wei, L. Teng, M. Fan, B. Li, L. Dong, L. Dong, Mn-modified CuO, CuFe2O4, and γ-Fe2O3 three-phase strong synergistic coexistence catalyst system for NO reduction by CO with a wider active window. ACS Appl. Mater. Interfaces 10, 40509–40522 (2018)
R.G. Crespo, A.Y. Al-Baitai, I. Saadoune, N.H. De Leeuw, Vacancy ordering and electronic structure of γ-Fe2O3 (maghemite): a theoretical investigation. J. Phys. 22, 255401 (2010)
A. Akbar, H. Yousaf, S. Riaz, S. Naseem, Role of precursor to solvent ratio in tuning the magnetization of iron oxide thin films–A sol-gel approach. J. Magn. Magn. Mater. 471, 14–24 (2019)
S. Babay, T. Mhiri, M. Toumi, Synthesis, structural and spectroscopic characterizations of maghemite γ-Fe2O3 prepared by one-step coprecipitation route. J. Mol. Struct. 1085, 286–293 (2015)
M. Mozaffari, S. Shatooti, M. Jafarzadeh, M. Niyaifar, A. Aftabi, H. Mohammadpour, S. Amiri, Synthesis of Zn2+ substituted maghemite nanoparticles and investigation of their structural and magnetic properties. J. Magn. Magn. Mater. 382, 366–375 (2015)
J. Ribis, Y. de Carlan, Interfacial strained structure and orientation relationships of the nanosized oxide particles deduced from elasticity-driven morphology in oxide dispersion strengthened materials. Acta Mater. 60, 238252 (2012)
R. Haul, T. Schoon, The structure of the ferromagnetic Iron (III) oxide γ-Fe2O3. Z. Phys. Chem. 44, 216–226 (1939)
P.B. Braun, A super structure in spinels. Nature 170, 1123 (1952)
G.W. Oosterhout, C.J.M. Rooijmans, A new superstructure in gamma-ferroic oxide. Nature 181, 44 (1958)
C. Greaves, A powder neutron diffraction investigation of vacancy ordering and covalence in γ-Fe2O3. J. Solid State Chem. 49, 325 (1983)
T.J. Bastow, A. Trinchi, M.R. Hill, R. Harris, T.H. Muster, Vacancy ordering in γ-Fe2O3 nanocrystals observed by 57FeNMR. J. Magn. Magn. Mater. 321, 2677–2681 (2009)
Z. Somogyvari, E. Svab, G. Meszaros, K. Krezhov, I. Nedkov, I. Sajo, F. Bouree, Vacancy ordering in nanosized maghemite fromneutron and X-ray powder diffraction. Appl. Phys. A 74, S1077–S1079 (2002)
H. Chen, Y. Zeng, W. Liu, S. Zhao, J. Wu, Y. Du, Multifaceted applications of nanomaterials in cell engineering and therapy. Biotechnol. Adv. 31, 638–653 (2013)
K. Yum, N. Wang, M.F. Yu, Nanoneedle: a multifunctional tool for biological studies in living cells. Nanoscale 2, 363–372 (2010)
J.G. Lu, P. Chang, Z. Fan, Quasi-one-dimensional metal oxide materials—synthesis, properties and applications. Mater. Sci. Eng. R 52, 49–91 (2006)
T. An, W.S. Choi, E. Lee, I. Kim, W. Moon, G. Lim, Fabrication of functional micro- and nanoneedles electrodes using a carbon nanotube template and electrodeposition. Nanoscale Res. Lett. 6, 306 (2011)
X. Sun, W. Liu, D. Ouyang, Synthesis of iron oxide nanoneedles and their field emission properties. J. Alloy Compd. 478, 38–40 (2009)
D.T. Mackay, M.T. Janish, U. Sahaym, P.G. Kotula, K.L. Jungjohann, C.B. Carter, M.G. Norton, Template-free electrochemical synthesis of tin nanostructures. J. Mater. Sci. 49, 1476–1483 (2014)
M. Mahdavi, M.B. Ahmad, M.J. Haron, F. Namvar, B. Nadi, M.Z.A. Rahman, J. Amin, Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 18, 7533–7548 (2013)
Y. Ge, C. Li, G.I. Waterhouse, X. Jiang, Z. Zhang, L. Yu, Polypyrrole/γ-Fe2O3/g-C3N4 nanocomposites for high-performance electromagnetic wave absorption. Synth. Metals 274, 116716 (2021)
S. Li, T. Zhang, R. Tang, H. Qiu, C. Wang, Z. Zhou, Solvothermal synthesis and characterization of monodisperse superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 379, 226–231 (2015)
L. Zhang, R. He, H.C. Gu, Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 253, 2611–2617 (2006)
D.K. Kim, Y. Zhang, W. Voit, K.V. Rao, M. Muhammed, Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J. Magn. Magn. Mater. 225, 30–36 (2001)
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L.V. Elst, R.N. Muller, Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008)
S. Riaz, S. Rehman, M. Abutalib, S. Naseem, Structural, optical, and dielectric properties of aluminum oxide nanofibers synthesized by a lower-temperature sol-gel approach. J. Electron. Mater. (2016). https://doi.org/10.1007/s11664-016-4754-4
S. Xuan, Y. Xiang, J. Wang, J.C. Yu, K.C.F. Leung, Tuning the grain size and particle size of superparamagnetic Fe3O4 microparticles. Chem. Mater. 21, 5079–5087 (2009)
I. Pribosic, D. Makovec, M. Drofenik, Formation of nanoneedles and nanoplatelets of KNbO3 perovskite during templated crystallization of the precursor gel. Chem. Mater. 17, 2953–2958 (2005)
C. Li, R. Wei, Y. Xu, A. Sun, L. Wei, Synthesis of hexagonal and triangular Fe3O4 nanosheets via seed-mediated solvothermal growth. Nano Res. 7, 536–543 (2014)
W. Wu, X. Xiao, S. Zhang, J. Zhou, L. Fan, F. Ren, C. Jiang, Large-Scale and controlled synthesis of iron oxide magnetic short nanotubes: shape evolution, growth mechanism, and magnetic properties. J. Phys. Chem. C 114, 16092–16103 (2010)
F. Jiao, J.C. Jumas, M. Womes, A.V. Chadwick, A. Harrison, P.G. Bruce, Synthesis of ordered mesoporous Fe3O4 and γ-Fe2O3 with crystalline walls using post-template reduction/oxidation. J. Am. Chem. Soc. 128, 12905–12909 (2006)
M.P. Morales, S.V. Verdaguer, M.I. Montero, C.J. Serna, Surface and internal spin canting in γ-Fe2O3 nanoparticles. Chem. Mater. 11, 3058–3064 (1999)
J. Tang, M. Myers, K.A. Bosnick, L.E. Brus, Magnetite Fe3O4 nanocrystals: spectroscopic observation of aqueous oxidation kinetics. J. Phys. Chem. B 107, 7501–7506 (2003)
S. Riaz, R. Ashraf, A. Akbar, S. Naseem, Free growth of iron oxide nanostructures by sol-gel spin coating technique—structural and magnetic properties. IEEE Trans. Magn. 50, 2301805 (2014)
A.G. Roca, M.P. Morales, C.J. Serna, Synthesis of monodispersed magnetite particles from different organometallic precursors. IEEE Trans. Magn. 40, 3025 (2006)
B.D. Cullity, Elements of X-ray Diffraction (Addison-Wesley Publishing Company, Boston, 1956)
L. Xu, G. Zheng, J. Miao, F. Xian, Dependence of structural and optical properties of sol–gel derived ZnO thin films on sol concentration. Appl. Surf. Sci. 258, 7760–7765 (2012)
S. Benramache, A. Arif, O. Belahssen, A. Guettaf, Study on the correlation between crystallite size and optical gap energy of doped ZnO thin film. J. Nanostruct. Chem. 3, 1–6 (2013)
I.S. Smolkova, N.E. Kazantseva, H. Parmar, V. Babayan, P. Smolka, P. Saha, Correlation between coprecipitation reaction course and magneto-structural properties of iron oxide nanoparticles. Mater. Chem. Phys. 155, 178–190 (2015)
D.J. Craik, Magnetic Oxides (Wiley, Hoboken, 1975)
J.A. Cuenca, K. Bugler, S. Taylor, D. Morgan, P. Williams, J. Bauer, A. Porch, Study of the magnetite to maghemite transition using microwave permittivity and permeability measurements. J. Phys. 28, 106002 (2016)
T. Belin, N. Millot, F. Villieras, O. Bertrand, J.P. Bellat, Structural variations as a function of surface adsorption in nanostructured particles. J. Phys. Chem. B 108, 5333–5340 (2004)
T. Belin, N.G. Millot, T. Caillot, D. Aymes, J.C. Niepce, Influence of grain size, oxygen stoichiometry, and synthesis conditions on the γ-Fe2O3 vacancies ordering and lattice parameters. J. Solid State Chem. 163, 459–465 (2002)
T.J. Daou, J.M. Greneche, G. Pourroy, S. Buathong, A. Derory, C.U. Bouillet, B. Donnio, D. Guillon, S.B. Colin, Coupling agent effect on magnetic properties of functionalized magnetite-based nanoparticles. Chem. Mater. 20, 5869–5875 (2008)
S.K. Sahoo, K. Agarwal, A.K. Singh, B.G. Polke, K.C. Raha, Characterization of γ- and α-Fe2O3 nano powders synthesized by emulsion precipitation-calcination route and rheological behaviour of α-Fe2O3. Int. J. Eng. Sci. Technol. 2, 118–126 (2010)
M. Raileanu, L. Todan, D. Crisan, N. Dragan, M. Crisan, C. Stan, C. Andronescu, M. Voicescu, B.S. Vasile, A. Ianculescu, Sol–gel zirconia nanopowders with α-cyclodextrin as organic additive. J. Alloy Compd. 517, 157–163 (2012)
L.V. Gasparov, D.B. Tanner, Infrared and Raman studies of the Verwey transition in magnetite. Phys. Rev. B. 62, 7939–7944 (2000)
I. Chamritski, G. Burns, Infrared- and Raman-active phonons of magnetite, maghemite, and hematite: a computer simulation and spectroscopic study. J. Phys. Chem. B 109, 4965–4968 (2005)
M.B. Yazdi, K.Y. Choi, D. Wulferding, P. Lemmens, L. Alff, Raman study of the Verwey transition in magnetite thin films. New J. Phys. 15, 103032 (2013)
A.M. Jubb, H.C. Allen, Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. Appl. Mater. Interface 2, 2804–2812 (2010)
K. Ali, A.K. Sarfraz, I.M. Mirza, A. Bahadur, S. Iqbal, A. Haq, Preparation of superparamagnetic maghemite (γ-Fe2O3) nanoparticles by wet chemical route and investigation of their magnetic and dielectric properties. Curr. Appl. Phys. 15, 925–929 (2015)
P.B. Shete, R.M. Patil, B.M. Tiwale, S.H. Pawar, Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 377, 406–410 (2015)
K. Kluchova, R. Zboril, J. Tucek, M. Pecova, L. Zajoncova, I. Safarik, M. Mashlan, I. Markova, D. Jancik, M. Sebela, H. Bartonkova, V. Bellesi, P. Novak, D. Petridis, Superparamagnetic maghemite nanoparticles from solid-state synthesis—their functionalization towards peroral MRI contrast agent and magnetic carrier for trypsin immobilization. Biomaterials 30, 2855–2863 (2009)
J. Lu, X. Jiao, D. Chen, W. Li, Solvothermal synthesis and characterization of Fe3O4 and γ-Fe2O3 nanoplates. J. Phys. Chem. C 113, 4012–4017 (2009)
R.H. Fowler, L. Nordheim, Electron emission in intense electric fields. Proc. R. Soc. Lond. 119, 173–181 (1928)
E.L. Murphy, R.H. Good Jr., Thermionic emission, field emission, and the transition region. Phys. Rev. 102(6), 1464 (1956)
D. Banerjee, A. Jha, K.K. Chattopadhyay, Low-temperature synthesis of amorphous carbon nanoneedle and study on its field emission property. Phys. E 41, 1174–1178 (2009)
Author information
Authors and Affiliations
Contributions
ZHS contributed to synthesis and preparation of the initial draft. AA contributed to synthesis and structural data analysis. SR performed research design and overall supervision. SSH performed magnetic analysis. RS contributed to XRD results. ZNK contributed to FTIR and electronic results. SN performed overall supervision and preparation of the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, Z.H., Awan, A., Riaz, S. et al. Field emission properties and ferromagnetic exchange interactions in γ-Fe2O3 and Fe3O4 nanoneedles—oleic acid-assisted growth. J Mater Sci: Mater Electron 33, 4025–4042 (2022). https://doi.org/10.1007/s10854-021-07594-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07594-8