Abstract
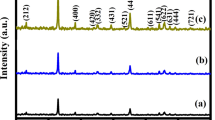
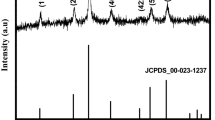
Utilizing Manganese Chloride tetra hydrate as a precursor, Pure and H2O2-assisted Mn3O4 were synthesized using a facile hydrothermal technique. The structural transformation was carried at room temperature by adding H2O2 to the precursor solution. The primary reactions of H2O2 in an alkaline medium, such as oxidative dissolution and moderate reducing activities are assists to modify the shape of the materials. The color of the mixture changes when H2O2 is added, and this is used to prove that the substance has undergone structural modification. It is directly visible through naked eyes. The crystal systems of the samples were analyzed through X-ray Diffraction. The surface morphology and an elemental composition of the samples were observed through scanning electron microscope and energy dispersive spectroscopy. Using Cyclic Voltammetry and Chronopotentiometry, the electrochemical characteristics of both samples were studied. In this chronopotentiometry analysis, H2O2 (Mn3O4) reveals an elevated specific capacitance of 1007 F/g at 0.1 A/g in 1 M aqueous Na2SO4 electrolyte. It has a potential window of 1.2 V, with a maximum energy density of 71 Wh/kg at a power density of 602 W/kg.











Similar content being viewed by others
Data availability
We have the original data and material with us.
Code availability
Not applicable.
References
J. Yan, T. Wei, W. Qiao, B. Shao, Q. Zhao, L. Zhang, Z. Fan, Rapid microwave-assisted synthesis of graphene nanosheet/Co3O4 composite for supercapacitors. Electrochim. Acta. 55(23), 6973–6978 (2010)
A. Gopalakrishnan, N. Vishnu, S. Badhulika, Cuprous oxide nanocubes decorated reduced graphene oxide nanosheets embedded in chitosan matrix: a versatile electrode material for stable supercapacitor and sensing applications. J. Electroanal. Chem. 834, 187–195 (2019)
C.R. Mariappan, V. Gajraj, S. Gade, A. Kumar, S. Dsoke, S. Indris, H. Ehrenberg, G.V. Prakash, R. Jose, Synthesis and electrochemical properties of rGO/polypyrrole/ferrites nanocomposites obtained via a hydrothermal route for hybrid aqueous supercapacitors. J. Electroanal. Chem. 845, 72–83 (2019)
S.H. Kazemi, M.A. Kiani, M. Ghaemmaghami, H. Kazemi, Nano-architectured MnO2 electrodeposited on the Cu-decorated nickel foam substrate as supercapacitor electrode with excellent areal capacitance. Electrochim. Acta 197, 107–116 (2016)
J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries, in Materials for sustainable energy a collection of peer-reviewed research and review articles from Nature Publishing Group. ed. by V. Dusastre (Co-Published with Macmillan Publishers Ltd, UK, 2011), pp. 171–179
J.R. Miller, P. Simon, Electrochemical capacitors for energy management. Sci. Magazine 321(5889), 651–652 (2008)
M. Winter, R.J. Brodd, What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104(10), 4245–4270 (2004)
J. Liu, Y. Li, X. Huang, R. Ding, Y. Hu, J. Jiang, L. Liao, Direct growth of SnO 2 nanorod array electrodes for lithium-ion batteries. J. Mater. Chem. 19(13), 1859–1864 (2009)
D. Chen, Q. Wang, R. Wang, G. Shen, Ternary oxide nanostructured materials for supercapacitors: a review. J. Mater. Chem. A 3(19), 10158–10173 (2015)
A. González, E. Goikolea, J.A. Barrena, R. Mysyk, Review on supercapacitors: technologies and materials. Renew. Sustain. Energy Rev. 58, 1189–1206 (2016)
J. Zhao, Z. Li, X. Yuan, T. Shen, L. Lin, M. Zhang, A. Meng, Q. Li, Novel core-shell multi-dimensional hybrid nanoarchitectures consisting of Co (OH) 2 nanoparticles/Ni3S2 nanosheets grown on SiC nanowire networks for high-performance asymmetric supercapacitors. Chem. Eng. Sci. 357, 21–32 (2019)
D. Zhang, Y. Shao, X. Kong, M. Jiang, X. Lei, Hierarchical carbon-decorated Fe3O4 on hollow CuO nanotube array: Fabrication and used as negative material for ultrahigh-energy density hybrid supercapacitor. Chem. Eng. Sci. 349, 491–499 (2018)
D.P. Dubal, O. Ayyad, V. Ruiz, P. Gomez-Romero, Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 44(7), 1777–1790 (2015)
D. Cericola, R. Kötz, Hybridization of rechargeable batteries and electrochemical capacitors: Principles and limits. Electrochim. Acta. 72, 1–17 (2012)
B. Shunmugapriya, A. Rose, T. Maiyalagan, T. Vijayakumar, Effect of cobalt doping on the electrochemical performance of trimanganese tetraoxide. Nanotechnology 31(28), 285401 (2020)
J. Bo, X. Luo, H. Huang, L. Li, W. Lai, X. Yu, Morphology-controlled fabrication of polypyrrole hydrogel for solid-state supercapacitor. J. Power Sources 407, 105–111 (2018)
S. Chang, J. Pu, J. Wang, H. Du, Q. Zhou, Z. Liu, C. Zhu, J. Li, H. Zhang, Electrochemical fabrication of monolithic electrodes with core/shell sandwiched transition metal oxide/oxyhydroxide for high-performance energy storage. ACS Appl. Mater. Interfaces 8(39), 25888–25895 (2016)
A.A. Yadav, S.N. Jadhav, D.M. Chougule, P.D. Patil, U.J. Chavan, Y.D. Kolekar, Spray deposited Hausmannite Mn3O4 thin films using aqueous/organic solvent mixture for supercapacitor applications. Electrochim. Acta 206, 134–142 (2016)
Y. Qiao, Q. Sun, J. Xi, H. Cui, Y. Tang, X. Wang, A modified solvothermal synthesis of porous Mn3O4 for supercapacitor with excellent rate capability and long cycle life. J. Alloys Compd 660, 416–422 (2016)
M. Aghazadeh, A. Bahrami-Samani, D. Gharailou, M.G. Maragheh, M.R. Ganjali, P. Norouzi, Mn3O4 nanorods with secondary plate-like nanostructures; preparation, characterization and application as high performance electrode material in supercapacitors. J. Mater. Sci. 27(11), 11192–11200 (2016)
L. Wang, G. Duan, S.M. Chen, X. Liu, Hydrothermally controlled synthesis of α-MnO2, γ-MnOOH, and Mn3O4 nanomaterials with enhanced electrochemical properties. J. Alloys Compd. 752, 123–132 (2018)
Z. Liu, Y. Xing, S. Fang, X. Qu, Facile synthesis of γ-MnOOH nanotubes and their application in electrochemical capacitors. J. Mater. Sci. 26(8), 5975–5979 (2015)
Z. Li, H. Bao, X. Miao, X. Chen, A facile route to growth of γ-MnOOH nanorods and electrochemical capacitance properties. J. Colloid Interf. Sci. 357(2), 286–291 (2011)
J. Yao, S. Yao, F. Gao, L. Duan, M. Niu, J. Liu, Reduced graphene oxide/Mn3O4 nanohybrid for high-rate pseduocapacitive electrodes. J. Colloid. Interf. Sci. 511, 434–439 (2018)
H. Jiang, T. Zhao, C. Yan, J. Ma, C. Li, Hydrothermal synthesis of novel Mn3O4 nano-octahedrons with enhanced supercapacitors performances. Nanoscale 2(10), 2195–2198 (2010)
J.W. Lee, A.S. Hall, J.D. Kim, T.E. Mallouk, A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem. Mater. 24(6), 1158–1164 (2012)
S. Ortaboy, J.P. Alper, F. Rossi, G. Bertoni, G. Salviati, C. Carraro, R. Maboudian, MnO x-decorated carbonized porous silicon nanowire electrodes for high performance supercapacitors. Energy Environ. Sci. 10(6), 1505–1516 (2017)
Y. Cheng, B. Li, Z. Wei, Y. Wang, D. Wei, D. Jia, Y. Feng, and Zhou, Y, Mn3O4 tetragonal bipyramid laden nitrogen doped and hierarchically porous carbon composite as positive electrode for high-performance asymmetric supercapacitor. J. Power Sources 451, 227775 (2020)
D.P. Shaik, R. Pitcheri, Y. Qiu, O.M. Hussain, Hydrothermally synthesized porous Mn3O4 nanoparticles with enhanced electrochemical performance for supercapacitors. Ceram 45(2), 2226–2233 (2019)
D. Wang, Y. Li, Q. Wang, T. Wang, Facile synthesis of porous Mn3O4 nanocrystal–graphene nanocomposites for electrochemical supercapacitors. Eur. J. Inorg. Chem. 2012, 628–635 (2012)
M. Tsuji, S. Gomi, Y. Maeda, M. Matsunaga, S. Hikino, K. Uto, T. Tsuji, H. Kawazumi, Rapid transformation from spherical nanoparticles, nanorods, cubes, or bipyramids to triangular prisms of silver with PVP, citrate, and H2O2. Langmuir 28(24), 8845–8861 (2012)
T. Parnklang, C. Lertvachirapaiboon, P. Pienpinijtham, K. Wongravee, C. Thammacharoen, S. Ekgasit, H2O2-triggered shape transformation of silver nanospheres to nanoprisms with controllable longitudinal LSPR wavelengths. RSC adv 3(31), 12886–12894 (2013)
G.S. Métraux, C.A. Mirkin, Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv. Mater 17(4), 412–415 (2005)
Q. Zhang, N. Li, J. Goebl, Z. Lu, Y. Yin, A systematic study of the synthesis of silver nanoplates: is citrate a “magic” reagent? J. Am. Chem. Soc. 133(46), 18931–18939 (2011)
R. Ranjithkumar, S.E. Arasi, N. Nallamuthu, P. Devendran, P. Lakshmanan, A. Arivarasan, M.K. Kumar, Investigation and fabrication of asymmetrical supercapacitor using nanostructured Mn3O4 immobilized carbon nanotube composite. Superlattice Microst. 138, 106380 (2020)
H. Wang, L. Pilon, Physical interpretation of cyclic voltammetry for measuring electric double layer capacitances. Electrochim Acta 64(130–139), 2012 (2012)
X. Zhang, P. Yu, D. Zhang, H. Zhang, X. Sun, Y. Ma, Room temperature synthesis of Mn3O4 nanoparticles: characterization, electrochemical properties and hydrothermal transformation to γ-MnO2 nanorods. Mater. Lett. 92, 401–404 (2013)
Y. Dai, K. Wang, J. Xie, From spinel Mn 3 O 4 to layered nanoarchitectures using electrochemical cycling and the distinctive pseudocapacitive behavior. Appl. Phys. Lett. 90(10), 104102 (2007)
S. Nagamuthu, S. Vijayakumar, G. Muralidharan, Ag incorporated Mn3O4/AC nanocomposite based supercapacitor devices with high energy density and power density. Dalton Trans. 43(46), 17528–17538 (2014)
V. Sannasi, K.U. Maheswari, C. Karthikeyan, S. Karuppuchamy, H2O2-assisted microwave synthesis of NiO/CNT nanocomposite material for supercapacitor applications. Ionics 26, 4067–4079 (2020)
K.T. Kubra, R. Sharif, B. Patil, A. Javaid, S. Shahzadi, A. Salman, S. Siddique, G. Ali, Hydrothermal synthesis of neodymium oxide nanoparticles and its nanocomposites with manganese oxide as electrode materials for supercapacitor application. J. Alloys Compd. 815, 152104 (2020)
E. Payami, A. Aghaiepour, K. Rahimpour, R. Mohammadi, R. Teimuri-Mofrad, Design and synthesis of ternary GO-Fc/Mn3O4/PANI nanocomposite for energy storage applications. J. Alloys Compd. 829, 154485 (2020)
G. Bharath, N. Arora, A. Hai, F. Banat, D. Savariraj, H. Taher, R.V. Mangalaraja, Synthesis of hierarchical Mn3O4 nanowires on reduced graphene oxide nanoarchitecture as effective pseudocapacitive electrodes for capacitive desalination application. Electrochim. Acta 337, 135668 (2020)
G. Jian, Y. Xu, L.C. Lai, C. Wang, M.R. Zachariah, Mn 3 O 4 hollow spheres for lithium-ion batteries with high rate and capacity. J. Mater. Chem. A 2(13), 4627–4632 (2014)
V.C. Bose, V. Biju, Mixed valence nanostructured Mn 3 O 4 for supercapacitor applications. Bull. Mater. Sci. 38(4), 865–873 (2015)
D. Prakash, C. Amente, K. Dharamvir, B. Singh, R. Singh, E.R. Shaaban, Y. Al-Douri, R. Khenata, M. Darroudi, K.D. Verma, Synthesis, purification and microstructural characterization of nickel doped carbon nanotubes for spintronic applications. Ceram. Int. 42(5), 5600–5606 (2016)
M. Guo, J. Balamurugan, N.H. Kim, J.H. Lee, High-energy solid-state asymmetric supercapacitor based on nickel vanadium oxide/NG and iron vanadium oxide/NG electrodes. Appl. Catal. B 239, 290–299 (2018)
J. Balamurugan, T.T. Nguyen, V. Aravindan, N.H. Kim, J.H. Lee, Flexible solid-state asymmetric supercapacitors based on nitrogen-doped graphene encapsulated ternary metal-nitrides with ultralong cycle life. Adv. Func. Mater. 28(44), 1804663 (2018)
J. Balamurugan, T.T. Nguyen, V. Aravindan, N.H. Kim, S.H. Lee, J.H. Lee, All ternary metal selenide nanostructures for high energy flexible charge storage devices. Nano Energy 65, 103999 (2019)
J. Balamurugan, C. Li, T.D. Thanh, O.K. Park, N.H. Kim, J.H. Lee, Hierarchical design of Cu 1–x Ni x S nanosheets for high-performance asymmetric solid-state supercapacitors. J. Mater. Chem. A 5(37), 19760–19772 (2017)
Acknowledgements
The authors thank SRM Central Instrumentation Facility (SCIF), and Nanotechnology Research Center (NRC), SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, India for the support in characterization studies. Financial support obtained from the Department of Space, Government of India (Grant No. B.19012/57/2016-II) through RESPOND project is gratefully acknowledged.
Funding
This work was not funded and no specific grant was received.
Author information
Authors and Affiliations
Contributions
The authors contributed to the design of the research work. Material synthesis, characterizations, and interpretation of the results were performed by BS. The manuscript was written by BS. The author TV pointed out major corrections and revised the manuscript. The authors read and accepted the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shunmugapriya, B., Vijayakumar, T. H2O2-assisted structural transformation of Mn3O4 nanoparticles to nanorods for supercapacitor applications. J Mater Sci: Mater Electron 33, 9334–9346 (2022). https://doi.org/10.1007/s10854-021-07300-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07300-8