Abstract
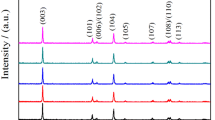
LiNi0.5−xCuxMn1.48Y0.02O4 (x = 0.02, 0.03, 0.04) and LiNi0.5Mn1.5O4 (LNMO) samples were prepared successfully via the sol–gel method. The lattice parameter and the degree of Ni/Mn disorder for LNMO samples were increased by doping Cu2+ and Y3+ ions, which is benefit for improving lithium-ion diffusion rate. The Cu–Y co-doped samples possessed truncated octahedral morphologies with (111) facet and exposed (100) facet by modified the co-doped Cu–Y content. The (100) facet helped to accelerate the Li+ ion diffusion while the (111) facet inhibited the dissolution of transition metals at the solid interface. The LiNi0.47Cu0.03Mn1.48Y0.02O4 (0.03 Cu–Y) sample exhibited high initial discharge specific capacity of 145.7 mAh g−1 which was far higher than that of pristine sample (113.8 mAh g−1). After 100 cycles at 1 C, the 0.03 Cu–Y sample retained discharge specific capacity of 137.2 mAh g−1 with superior retention of 96.79% while the undoped sample only retained 108.8 mAh g−1 and the retention is 95.79% at the same condition. The improved electrochemical property of the Cu–Y co-doped sample maybe attribute to the stable structure that decrease Ohmic polarization and the suitable morphology that is conducive to accelerate the Li+ ion diffusion.










Similar content being viewed by others
References
A. Manthiram, K. Chemelewski, E.S. Lee, A perspective on the high-voltage LiMn1.5Ni0.5O4 spinel cathode for lithium-ion batteries. Energy Environ. Sci. 7, 1339–1350 (2013)
P.Y. Guan, L. Zhou, Z.L. Yu, Y.D. Sun, Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Mater Chem. 43, 220–235 (2020)
G.J. Li, Y.H. Liao, Z.Y. He, H.B. Zhou, A new strategy to improve the cyclic stability of high voltage lithium nickel manganese oxide cathode by poly (butyl methacrylate-acrylonitrile-styrene) terpolymer as co-binder in lithium-ion batteries. Electrochim. Acta 319, 527–540 (2019)
Y. Luo, H.Y. Li, T. L. Lu, Y.X. Zhang, S.S. Mao, Z. Liu, W. Wen, J.Y. Xie, L.Q. Yan, Fluorine gradient-doped LiNi0.5Mn1.5O4 spinel with improved high voltage stability for Li-ion batteries. Electrochim. Acta 238, 237–245 (2017)
J.D. Duan, Y.L. Liu, X. Tang, J. Li, J.Q. Guo, Improve electrochemical performance of spinel LiNi0.5Mn1.5O4 via surface modified by Li1.2Ni0.2Mn0.6O2 layered materials. J. Mater. Sci.: Mater. Electron. 31, 4336–4344 (2020)
A.V. Potapenko, S.A. Kirillov, Enhancing high-rate electrochemical properties of LiMn2O4 in a LiMn2O4/LiNi0.5Mn1.5O4 core/shell composite. Electrochim. Acta 258, 9–16 (2017)
L. Li, Q. Liu, J.J. Huang, S.Y. Luo, Synthesis and electrochemical properties of Zn-doping LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion battery application. J. Mater. Sci.: Mater. Electron. 31, 12409–12416 (2020)
L. Li, J.S. Sui, J. Chen, Y.C. Lu, LiNi0.5Mn1.5O4 microrod with ultrahigh Mn3+ content: a high performance cathode material for lithium-ion battery. Electrochim. Acta 305, 433–442 (2019)
C.T. Chu, A. Mondal, N.V. Kosova, J.Y. Lin, Improved high-temperature cyclability of AlF3 modified spinel LiNi0.5Mn1.5O4 cathode for lithium-ion batteries. Appl. Surf. Sci. 530, 147169 (2020)
Y.P. Li, Q. Zhang, T.H. Xue, LaF3 nano layer surface modified spinel LiNi0.5Mn1.5O4 cathode material for advanced lithium-ion batteries. Ceram. Int. 44, 4058–4066 (2018)
D. Hong, Y.F. Guo, H.X. Wang, Mechanism for improving the cycle performance of LiNi0.5Mn1.5O4 by RuO2 surface modification and increasing discharge cut-off potentials. J. Mater. Chem. A 3, 15457–15465 (2015)
Q. Chang, A.J. Wei, W. Li, X. Bai, Structural and electrochemical characteristics of Al2O3-modified LiNi0.5Mn1.5O4 cathode materials for lithium-ion batteries. Ceram. Int. 45, 5100–5110 (2018)
T.H. Xu, Y.P. Li, D.D. Wang, Enhanced electrochemical performance of LiNi0.5Mn1.5O4 cathode material by YPO4 surface modification. ACS Sustain. Chem. Eng. 6, 5818–5825 (2018)
T.F. Yi, Y. Xie, M.F. Ye, Recent developments in the doping of LiNi0.5Mn1.5O4 cathode material for 5V lithium-ion batteries. Ionics 17, 383–389 (2011)
D.F. Zhou, J.Q. Li, C. Chen, F.C. Lin, H.M Wu, A hydrothermal synthesis of Ru-doped LiMn1.5Ni0.5O4 cathode materials for enhanced electrochemical performance. RSC Adv. 11, 12549 (2021)
Y. Lou, T.L. Lu, Y.X. Zhang, Surface-segregated, high-voltage spinel lithium-ion battery cathode material LiNi0.5Mn1.5O4 cathodes by aluminium doping with improved high-rate cyclability. J. Alloys Compd. 703, 289–297 (2017)
T.F. Yi, B. Chen, Y.R. Zhu, X.Y. Li, R.S. Zhu, Enhanced rate performance of molybdenum-doped spinel LiNi0.5Mn1.5O4 cathode materials for lithium ion battery. J. Power Sources 247, 778–785 (2014)
T.F. Yi, Y. Xie, Y.R. Zhu, R.S. Zhu, M.F. Ye, High rate micron-sized niobium-doped LiMn1.5Ni0.5O4 as ultra high power positive-electrode material for lithium-ion batteries. J. Power Sources 211, 59–65 (2012)
B. Zong, Y.Q. Lang, S.H. Yan, Z.Y. Deng, J.J. Gong, J.L. Guo, L. Wang, G.C. Liang, Influence of Ti doping on microstructure and electrochemical performance of LiNi0.5Mn1.5O4 cathode material for lithium-ion batteries. Mater. Today Commun. 24, 101003 (2020)
H.Y. Sun, X. Kong, S.P. Feng, G.Y. Liu, Effects of Zn doping amount on the electrochemical properties of LiNi0.5Mn1.5O4 lithium-ion cathode materials. Int. J. Electrochem. Sci. 14, 11391–11405 (2019)
W. Wu, J.L. Guo, X. Qin, C.B. Bi, Enhanced electrochemical performances of LiNi0.5Mn1.5O4 spinel in half-cell and full-cell via yttrium doping. J. Alloys Compd. 721, 721–730 (2017)
H.Y. Sun, X. Kong, B.S. Wang, T.B. Luo, G.Y. Liu, Cu doped LiNi0.5Mn1.5−xCuxO4 (x = 0, 0.03, 0.05, 0.10, 0.15) with significant improved electrochemical performance prepared by a modified low temperature solution combustion synthesis method. Ceram. Int. 44, 4603–4610 (2018)
A. Milewska, L. Kondracki, M. Molenda, J. Molenda, Structural, transport and electrochemical properties of LiNi0.5−yCuyMn1.5O4−δ spinel cathode materials. Solid State Ion. 267, 27–31 (2014)
J.J. Pan, B. Chen, Y. Xie, N. Ren, T.F. Yi, V2O5 modified LiNi0.5Mn1.5O4 as cathode material for high-performance Li-ion battery. Mater. Lett. 253, 136–139 (2019)
B. Yun, J. Hong, M.G. Kim, Large-scale synthesis of high electrochemical performance LiNi0.5Mn1.5O4 by using a polyvinylpyrrolidone-assisted citric-acid sol–gel combustion method. J. Korean Phys. Soc. 77, 332–336 (2020)
O. Sha, Z. Qiao, S.L. Wang, Z.Y. Tang, Improvement of cycle stability at elevated temperature and high rate for LiNi0.5−xCuxMn1.5O4 cathode material after Cu substitution. Mater. Res. Bull. 48, 1606–1611 (2013)
L.Z. Xiong, W.P. Liu, Y.X. Wu, Z.Q. He, Synthesis and characterization of LiNi0.49Mn1.49Y0.02O4@Ag by electroless plating technique. Appl. Surf. Sci. 328, 531–535 (2015)
K.R. Chemelewski, A. Manthiram, Origin of site disorder and oxygen non-stoichiometry in LiMn1.5Ni0.5–xMxO4 (M = Cu and Zn) cathodes with divalent dopant ions. J. Phys. Chem. C 117, 12465–12471 (2013)
J.F. Wang, D. Chen, W. Wu, L. Wang, G.C. Liang, Effects of Na+ doping on crystalline structure and electrochemical performances of LiNi0.5Mn1.5O4 cathode material. Trans. Nonferrous Met. Soc. China 27, 2239–2248 (2017)
S.H. Oh, K.Y. Chung, S.H. Jeon, C.S. Kim, W.I. Cho, B.W. Cho, Structural and electrochemical investigations on the LiNi0.5−xMn1.5−yMx+yO4 (M = Cr, Al, Zr) compound for 5V cathode material. J. Alloys Compd. 469, 244–250 (2009)
A.V. Potapenko, S.A. Kirillov, Lithium manganese spinel materials for high-rate electrochemical applications. J. Energy Chem. 23, 543–558 (2014)
P. Sun, Y. Ma, T.Y. Zhai, H.Q. Li, High performance LiNi0.5Mn1.5O4 cathode by Al-coating and Al3+-doping through a physical vapor deposition method. Electrochim. Acta 191, 237–246 (2016)
J.C. Deng, Y.L. Xu, L.L. Xiong, Improving the fast discharge performance of high-voltage LiNi0.5Mn1.5O4 spinel by Cu2+, Al3+, Ti4+ tri-doping. J. Alloys Compd. 677, 18–26 (2016)
G.Y. Liu, X. Kong, H. Sun, B. Wang, A facile template method to synthesize significantly improved LiNi0.5Mn1.5O4 using corn stalk as a bio-template. Electrochim. Acta 141, 141141–141148 (2014)
J.S. Park, K.C. Roh, J.W. Lee, Structurally stabilized LiNi0.5Mn1.5O4 with enhanced electrochemical properties through nitric acid treatment. J. Power Sources 230, 138–142 (2013)
M.C. Kim, Y.W. Lee, T.K. Pham, J.I. Sohn, K.W. Park, Chemical valence electron-engineered LiNi0.4Mn1.5MtO4 (Mt = Co and Fe) cathode materials with high-performance electrochemical properties. Appl. Surf. Sci. 504, 144514 (2020)
S.Y. Li, Y. Wei, P. Wang, Y.H. Feng, W.B. Liang, H. Ding, X.L. Cui, Synergism of Cu and Al co-doping on improvements of structural integrity and electrochemical performance for LiNi0.5Mn1.5O4. J. Alloys Compd. 820, 153140 (2020)
T.F. Yi, J.P. Qu, X. Lai, X. Han, H. Chang, Y.R. Zhu, Toward high-performance Li storage anodes: design and construction of spherical carbon-coated CoNiO2 materials. Mater. Today Chem. 19, 100407 (2021)
T.F. Yi, L.Y. Qiu, J. Mei, S.Y. Qi, P. Cui, S.H. Luo, Y.R. Zhu, Y. Xie, Y.B. He, Porous spherical NiO@NiMoO4@PPy nanoarchitectures as advanced electrochemical pseudocapacitor materials. Sci. Bull. 65, 546–556 (2020)
X.G. Hao, M.H. Austin, B.M. Bartlett, Two-step hydrothermal synthesis of submicron Li1+xNi0.5Mn1.5O4−δ for lithium-ion battery cathodes (x = 0.02, δ = 0.12). Dalton Trans. 41, 8067 (2012)
A. Wei, J.P. Mu, R. He, X. Bai, Z. Liu, Enhancing electrochemical performance and structural stability of LiNi0.5Mn1.5O4 cathode material for rechargeable lithium-ion batteries by boron doping. Ceram. Int. 47, 226–237 (2021)
C.Y. Yu, L. Dong, Y.X. Zhang, Promoting electrochemical performances of LiNi0.5Mn1.5O4 cathode via YF3 surface coating. Solid State Ion. 357, 115464 (2020)
Y.P. Li, D.D. Wang, T.H. Xu, M.Y. Wu, Stabilized structural and electrochemical properties of LiNi0.5Mn1.5O4 via ZrF4 nanolayer modification for Li-ion batteries. Solid State Ion. 324, 7–12 (2018)
A.J. Wei, W. Li, Q. Chang, X. Bai, R. He, L.H. Zhang, Effect of Mg2+/F co-doping on electrochemical performance of LiNi0.5Mn1.5O4 for 5V lithium-ion batteries. Electrochim. Acta 323, 134692 (2019)
C. Zhan, T.P. Wu, J. Lu, K. Amine, Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes—a critical review. Energy Environ. Sci. 11, 243–257 (2018)
M.Y. Mo, K.S. Hui, X.T. Hong, J.S. Guo, C.C. Ye, A.J. Li, N.Q. Hu, Z.Z. Huang, J.H. Jiang, J.Z. Liang, H.Y. Chen, Improved cycling and rate performance of Sm-doped LiNi0.5Mn1.5O4 cathode materials for 5V lithium ion batteries. Appl. Surf. Sci. 290, 412–418 (2014)
C.Y. Zhu, Y.N. Zhang, X.H. Yu, P. Dong, J.G. Duan, J.M. Liu, J.X. Liu, Y.J. Zhang, Controllable fabrication and Li storage kinetics of 1D spinel LiMn2O4 positive materials for Li-ion batteries: an exploration of critical diameter. ChemSusChem 13, 803–810 (2020)
C.Y. Zhu, J.X. Liu, X.H. Yu, Y.J. Zhang, P. Dong, X. Wang, Boosting the stable Li storage performance in one-dimensional LiLaxMn2−xO4 nanorods at elevated temperature. Ceram. Int. 45, 19351–19359 (2019)
Acknowledgements
Authors gratefully acknowledge financial support from Science and Technology Project of Guizhou (2016/5667) and (2021488), Science and Technology Foundation of Guizhou Province (2019/5635, Natural Science Foundation of China (52063005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, F., Wu, H., Chen, T. et al. The Cu–Y co-doping LiNi0.5Mn1.5O4 with modified morphology and enhanced electrochemical property for a 5 V lithium-ion battery. J Mater Sci: Mater Electron 33, 283–297 (2022). https://doi.org/10.1007/s10854-021-07292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07292-5