Abstract
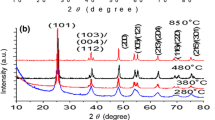
Phase pure stannic oxide (SnO2) is an efficient and reliable anode material for Li ion batteries. Understanding of pure SnO2 phase formation with respect to different calcination temperatures (200 °C, 300 °C, 400 °C, 600 °C, and 800 °C) is attempted in the present work. The samples are prepared by precipitation method and subjected for structural analysis which exhibit varied percentages of crystallinity and crystallite size (17–40 nm) with respect to their calcination temperature varying from 200 to 800 °C. Thermal analysis reveal that presence of tin hydroxide composition is unavoidable and formation of pure SnO2 is possible only after 600 °C, up to which small amount of weight loss is seen in all the samples. Morphological analyses reveal the spherical grain distribution and grains are dispersed well for samples calcined at high temperatures. Cyclic voltammetry analysis expose that SnO2 with high crystallinity/free from impurity traces are better at electrochemical properties. Also, SnO2 calcined at 800 °C exhibit better redox reactions and good cycling ability up to 500 cycles. The charge–discharge analysis shows better specific capacitance for this material, ~ 160 mAhg−1 in aqueous electrochemical system. On the other hand, electrical conductivity of the sample is 1.9 × 10–4 Scm−1 at room temperature as studied by AC impedance spectroscopy.












Similar content being viewed by others
References
J. Ma, J. Sung, J. Hong, S. Chae, N. Kim, S.H. Choi, G. Nam, Y. Son, S.Y. Kim, M. Ko, J. Cho, Towards maximized volumetric capacity via porecoordinated design for large-volume-change lithium-ion battery anodes. Nat. Comm. 10, 475 (2019). https://doi.org/10.1038/s41467-018-08233-3
Y.M. Chang, H.W. Lin, L.-J. Li, H.Y. Chen, Two-dimensional materials as anodes for sodium-ion batteries. Mater. Today Adv. 6, 100054 (2020)
S.H. Yu, X. Feng, N. Zhang, J. Seok, H.D. Abruña, Abrun, Understanding conversion-type electrodes for lithium rechargeable batteries. Acc Chem Res Special issue: Energy Storage: complexities among materials and interfaces at multiple length scales. 6, 100054 (2020). https://doi.org/10.1021/acs.accounts.7b00487
J.S. Chen, L.A. Archer, X.W. Lou, SnO2 hollow structures and TiO2 nanosheets for lithium-ion batteries. J. Mater. Chem. 21, 9912–9924 (2011). https://doi.org/10.1039/C0JM04163G
F. Zoller, D. Bçhm, T. Bein, D. Fattakhova-Rohlfing, Tin oxide based nanomaterials and their application as anodes in lithium-ion batteries and beyond. ChemSusChem Minireviews 12, 4140–4159 (2019)
Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa, T. Miyasaka, Tin- based amorphous oxide: a high- capacity lithium ion storage material. Science 276, 1395–1397 (1997)
Bo. Zhao, A. Dhara, J. Dendooven, C. Detavernier, Atomic layer deposition of SnO2-based composite anodes for thin-film lithium-ion batteries. Front. Energy Res. 8, 609417 (2020). https://doi.org/10.3389/fenrg.2020.609417
D. Dixon, M. Ávila, H. Ehrenberg, A. Bhaskar, Difference in electrochemical mechanism of SnO2 conversion in lithium-ion and sodium-ion batteries: combined in operando and ex situ XAS investigations. ACS Omega 4, 9731–9738 (2019)
J.P. Pender, G. Jha, D.H. Youn, J.M. Ziegler, I. Andoni, E.J. Choi, A. Heller, B.S. Dunn, P.S. Weiss, R.M. Penner, C.B. Mullins, Electrode degradation in lithium-ion batteries. ACS Nano 14–2, 1243–1295 (2020). https://doi.org/10.1021/acsnano.9b04365
D. Hernandez, F. Mendoza, E. Febus, B.R. Weiner, G. Morell, Binder free SnO2-CNT composite as anode material for Li-ion battery. J. Nanotechnol. (2014). https://doi.org/10.1155/2014/381273
J. Cui, S. Yao, J.Q. Huang, L. Qin, W.G. Chong, Z. Sadighi, J. Huang, Z. Wang, J.K. Kim, Sb-doped SnO2/graphene-CNT aerogels for high performance Li-ion and Na-ion battery anodes. Energy Storage Mater. 9, 85–95 (2017)
Wu. Ping, Du. Ning, H. Zhang, Yu. Jingxue, D. Yang, CNTs@SnO2@C coaxial nanocables with highly reversible lithium storage. J. Phys. Chem. C 114, 51 (2010)
J. Guo, P. Li, L. Chai, Su. Yi, J. Diao, X. Guo, Silica template-assisted synthesis of SnO2@porous carbon composites as anode materials with excellent rate capability and cycling stability for lithium-ion batteries. RSC Adv. 7, 30070 (2017). https://doi.org/10.1039/c7ra03594b
S. Jayapandi, S. Premkumar, D. Lakshmi, P. Packiyaraj, P. Sivaraj, K. Anitha, Reinforced photocatalytic reduction of SnO2 nanoparticle by La incorporation for efficient photodegradation under visible light irradiation. J. Mater. Sci. 30, 8479–8492 (2019)
A. Pramanik, S. Maiti, S. Mahanty, Metal hydroxides as a conversion electrode for lithium-ion batteries: a case study with a Cu(OH)2 nanoflower array. J. Mater. Chem. A 2, 18515–18522 (2014)
J.S. Dias, F.R.M. Batista, R. Bacani, E.R. Tribo, Structural characterization of SnO nanoparticles synthesized by the hydrothermal and microwave routes. Sci. Rep. 10, 9446 (2020). https://doi.org/10.1038/s41598-020-66043-4
S. Khalameida, M. Samsonenko, J. Skubiszewska-Zieba, O. Zakutevskyy, Dyes catalytic degradation using modified tin(IV) oxide and hydroxide powders. Adsorpt. Sci. Technol. Special Collection—15th Ukrainian-Polish Symposium, (2017) https://doi.org/10.1177/0263617417722251
G. PaParoni, D. Walker, J.D. Webster, Cassiterite-saturated minimum melting behavior within Sn-SnO2-SiO2 at 1 atm and 10 kbar. Am. Miner. 95, 784–798 (2010). https://doi.org/10.2138/am.2010.3319
D. Vasanth Raj, N. Ponpandian, D. Mangalaraj, C. Viswanathan, Electrochemical behavior of nanostructured SnO2 thin films in aqueous electrolyte solutions. Mater. Sci. Semicond. Process. 26, 55–61 (2014)
Y. Cheng, A. Nie, L.-Y. Gan, Q. Zhang, U. Schwingenschlogl, A global view of the phase transitions of SnO2 in rechargeable batteries based on results of high throughput calculations. J. Mater. Chem. A 3, 19483–19489 (2015)
A. Lakshmi Narayana, M. Dhananjaya, N. Guru Prakash, A. Mauger, C.M. Julien, O.M. Hussain, Li2TiO3/Ni foam composite as high-performance electrode for energy storage and conversion. Heliyon 5(7), e02060 (2019). https://doi.org/10.1016/j.heliyon.2019.e02060
S. Wu, M. Wang, C. Li, Y. Zhu, H. Wang, Single crystalline SnO2 nanowires obtained from heat-treated SnO2 and C mixture and their electrochemical properties. Mater. Chem. Phys. (2014). https://doi.org/10.1016/j.matchemphys.2014.04.028
D. Dixon, M. Ávila, H. Ehrenberg, A. Bhaskar, Difference in electrochemical mechanism of SnO2 conversion in lithium-ion and sodium-ion batteries: combined in operando and ex situ XAS investigations. ACS Omega 4(6), 9731–9738 (2019)
A.B. Kuriganova, C.A. Vlaic, S. Ivanov, D.V. Leontyeva, A. Bund, N.V. Smirnova, Electrochemical dispersion method for the synthesis of SnO2 as anode material for lithium ion batteries. J. Appl. Electrochem. 46, 527–538 (2016)
M.V. Reddy, L.Y. Andreea, A.Y. Ling, J.N. Hwee, C.A. Lin, S. Admas, K.P. Loh, M.K. Mathe, K.I. Ozoemena, B.V. Chowdari, Effect of preparation temperature and cycling voltage range on molten salt method prepared SnO2. Electrochim. Acta 106, 143–148 (2013)
Z.P. Guo, G.D. Du, Y. Nuli, M.F. Hassan, H.K. Liu, Ultra-fine porous SnO2 nanopowder prepared via a molten salt process: a highly efficient anode material for lithium-ion batteries. J. Mater. Chem. 19, 3253–3257 (2009). https://doi.org/10.1039/B821519G
R. Liu, W.D. Yang, Y.J. Song, C. Liu, Calcination temperature effects on the architecture, morphology and discharge properties of SnO2 electrode materials Ge performances of a LiNi0.8Co0.2O2 electrode material. J. Anal. Bioanal. Tech. 8, 382 (2017). https://doi.org/10.4172/2155-9872.1000382
D. Lakshmi, B. Nalini, P. Sivaraj, S. Jayapandi, Electro analytical studies on indium incorporated SnSb alloy anode for Li-ion batteries. J. Electroanal. Chem. 801, 459–465 (2017)
W.B. Soltan, S. Nasri, M.S. Lassoued, S. Ammar, Structural, optical properties, impedance spectroscopy studies and electrical conductivity of SnO2 nanoparticles prepared by polyol method. J. Mater. Sci. 28, 6649–6656 (2017). https://doi.org/10.1007/s10854-017-6356-1
Acknowledgements
Dr. D. Lakshmi acknowledges Council for Scientific & Industrial Research, Government of India for the financial assistance through research associateship [09/472(0183)/219-EMR-I]. Ms. P. Priyanka acknowledges Department of Science and Technology (DST)-INSPIRE fellowship [IF180415], Government of India for the financial assistance. Prof. P. Christopher Selvin acknowledges DST-Science and Engineering Research Board for the financial aid under core research grant [CRG/2020/004896].
Funding
Dr. D. Lakshmi acknowledges CSIR, Government of India for the financial assistance through research associateship [09/472(0183)/219-EMR-I]. Ms. P. Priyanka acknowledges DST-INSPIRE fellowship [IF180415], Government of India for the financial assistance. Prof. P. Christopher Selvin acknowledges DST-SERB for the financial aid under core research grant [CRG/2020/004896].
Author information
Authors and Affiliations
Contributions
DL: Conceptualization, Formal analysis, writing original draft. MID: Investigation. BN: Supervision. GGS: Investigation. PP: Investigation. SJ: Resources. PCS: Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest is declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakshmi, D., Infanta Diana, M., Nalini, B. et al. Fine-tuning of stannic oxide anodes’ material properties through calcination. J Mater Sci: Mater Electron 32, 27384–27397 (2021). https://doi.org/10.1007/s10854-021-07114-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07114-8