Abstract
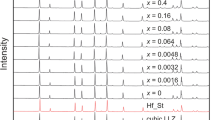
New triclinic oxide material Ta1−x−yTixCryO2.5−δ (x = 0.077, y = 0–0.053) was synthesized via an oxalate co-precipitation method and tested as a novel electrolyte for intermediate-temperature solid oxide fuel cells (IT-SOFCs). Structural, electrical, and thermal properties of tantalum-based electrolyte co-doping with Cr3+/Ti4+ content were studied. X-ray powder diffraction showed that the crystal structure changed from orthorhombic to triclinic due to co-doped, and its high-temperature triclinic (pseudo-tetragonal) phase of tantalum pentoxide (H-Ta2O5) structure was stabilized to room temperature by doping. The crystalline phase facilitates the generation of more oxygen vacancies, which increases the electrical conductivity. The appearance of oxygen vacancies in the crystal with triclinic structure was confirmed with Raman spectroscopy and X-ray photoelectron spectroscopy. Scanning electron microscopy results exhibited the grain size decreases gradually with doping. Impedance curves showed high total ionic conductivity (1.48 × 10–1 S/cm at 700 °C) and low activation energy (Ea = 0.857 eV) for Ta0.9Ti0.067Cr0.033O2.5−δ. Thermal expansion coefficients (3.09 × 10–6 K−1) for co-doped samples were much lower in comparison to other electrolytes. Based on the results reported in this work, Ta1−x−yTixCryO2.5−δ can be an excellent oxygen conductor and recommended as solid electrolytes for IT-SOFC applications.










Similar content being viewed by others
References
A. Lashtabeg, S.J. Skinner, Solid oxide fuel cells—a challenge for materials chemists? J. Mater. Chem. 16(31), 3161–3170 (2006)
T. Yang, H. Zhao, M. Fang, K. Swierczek, J. Wang, Z. Du, A new family of Cu-doped lanthanum silicate apatites as electrolyte materials for SOFCs: synthesis, structural and electrical properties. J. Eur. Ceram. Soc. 39, 424–431 (2018)
C. Madhusudan, K. Venkataramana et al., Structural, electrical and thermal studies on microwave sintered Dy and Pr co-doped ceria ceramics as electrolytes for intermediate temperature solid oxide fuel cells. J. Mater. Sci.: Mater. Electron. 29(19), 17067–17077 (2018)
M.A. Borik, S.I. Bredikhin, V.T. Bublik et al., The impact of structural changes in ZrO2-Y2O3 solid solution crystals grown by directional crystallization of the melt on their transport characteristics. Mater. Lett. 205, 186–189 (2017)
E.D. Wachsman, K.T. Lee, Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011)
P. Satardekar, D. Montinaro, V.M. Sglavo, Fe-doped YSZ electrolyte for the fabrication of metal supported-SOFC by co-sintering. Ceram. Int. 41(8), 9806–9812 (2015)
X. Wang, T. Liu, C. Wang et al., Crystalline structure, microstructure and electrical characterizations of FeO1.5 doped YSZ. Ceram. Intl. 43(12), 9577–9581 (2017)
Q. Zhou, W.C.J. Wei, Y. Guo et al., LaSrMnCoO5+δ as cathode for intermediate-temperature solid oxide fuel cells. Electrochem. Commun. 19, 36–38 (2012)
Y. Chen, J. Xu, S. Xie et al., Ion doping effects on the lattice distortion and interlayer mismatch of aurivillius-type bismuth titanate compounds. Materials 11(5), 821 (2018)
H. Zhang, L. Zhao et al., Oxygen diffusion and electrochemical performance of La0.6−xSr0.4BaxCo1−yFeyO3−δ. J. Mater. Sci. 30(22), 20050–20057 (2019)
R.K. Raghvendra, P. Singh, Electrical conductivity of barium substituted LSGM electrolyte materials for IT-SOFC. Solid State Ionics 262, 428–432 (2014)
P. Gao, A. Bolon, M. Taneja et al., Thermal expansion and elastic moduli of electrolyte materials for high and intermediate temperature solid oxide fuel cell. Solid State Ionics 300, 1–9 (2017)
Z. Song, L. Yi, C. Li et al., Synthesis & characterization of Ti and Fe co-doped Ta2O5 based solid electrolytes for SOFC. Solid State Ionics 312, 106–111 (2017)
S. Xia, J. Ni, S.V. Savilov, L. Li, Oxygen-deficient Ta2O5 nanoporous films as self-supported electrodes for lithium microbatteries. Nano Energy. 45, 407–412 (2018)
X. Yu, W. Li, J. Huang, Z. Li, J. Liu, P. Hu, Superstructure Ta2O5 mesocrystals derived from (NH4)2Ta2O3F6 mesocrystals with efficient photocatalytic activity. Dalton Trans. 47(6), 1948–1957 (2018)
P.S. Dobal, R.S. Katiyar, Y. Jiang, R. Guo, A.S. Bhalla, Micro-Raman scattering and X-ray diffraction studies of (Ta2O5)1–x(TiO2)x ceramics. J. Appl. Phys. 87(12), 8688–8694 (2000)
Y.-L. Chueh, L.-J. Chou, Z.L. Wang, SiO2/Ta2O5 core-shell nanowires and nanotubes. Angew. Chem. 118(46), 7937–7942 (2006)
Q. Shi, T. Li et al., Synthesis and ionic conductivities of M (Mg, Ba, Zr) and Al co-doped apatite-type lanthanum germanate electrolytes for IT-SOFC. J. Mater. Sci. 29(4), 2725–2732 (2018)
M. Jayaratna, M. Yoshimura et al., Electrical conductivity of Cr2O3-doped Y2O3-stabilized ZrO2. J. Mater. Sci. 22, 2011–2016 (1987)
K. Amarsingh Bhabu, J. Theerthagiri et al., Cubic fluorite phase of samarium doped cerium oxide (CeO2)0.96Sm0.04 for solid oxide fuel cell electrolyte. J. Mater. Sci. 272, 1566–1573 (2016)
U. Holzwarth, N. Gibson, The Scherrer equation versus the 'Debye-Scherrer equation'. Nat. Nanotechnol. 6(9), 534 (2011)
U.N. Gries, H. Schraknepper, K. Skaja, F. Gunkel, S. Hoffmann-Eifert, R. Waser, R.A. De Souza, A SIMS study of cation and anion diffusion in tantalum oxide. Phys. Chem. Chem. Phys. 20(2), 989–996 (2018)
C. Zhao, R. Liu, S. Wang et al., Fabrication of a large area cathode-supported thin electrolyte film for solid oxide fuel cells via tape casting and co-sintering techniques. Electrochem. Commun. 11(4), 842–845 (2009)
T.K. Pietrzak, L. Wewior, J.E. Garbarczyk, M. Wasiucionek, I. Gorzkowska, J.L. Nowinski, S. Gierlotka, Electrical properties and thermal stability of FePO4 glasses and nanomaterials. Solid State Ionics 188(1), 99–103 (2011)
E.E. Nikishina, E.N. Lebedeva, D.V. Drobot, Niobium- and tantalum-containing oxide materials: synthesis, properties, and application. Inorg. Mater. 48(13), 1243–1260 (2012)
I.E. Grey, W.G. Mumme, R.S. Roth, The crystal chemistry of L-Ta2O5 and related structures. J. Solid State Chem. 178(11), 3308–3314 (2005)
X.Q. Liu, X.D. Han, Z. Zhang et al., The crystal structure of high temperature phase Ta2O5. Acta Mater. 55(7), 2385–2396 (2007)
P. Wynblatt, G.S. Rohrer, F. Papillon, Grain boundary segregation in oxide ceramics. J. Eur. Ceram. Soc. 23(15), 2841–2848 (2003)
S. Hui, J. Roller, S. Yick et al., A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J. Power Sources 172(2), 493–502 (2007)
B.H. Smith, W.C. Holler, M.D. Gross, Electrical properties and redox stability of tantalum-doped strontium titanate for SOFC anodes. Solid State Ionics 192(1), 383–386 (2011)
Y. Chen, Z. Pen, Q. Wang et al., Crystalline structure, ferroelectric properties, and electrical conduction characteristics of W/Cr co-doped Bi4Ti3O12 ceramics. J. Alloy Compd. 612, 120–125 (2014)
R. Bredesen, P. Kofstad, A reinterpretation of the defect structure of L-Ta2O5. Solid State Ionics 27, 11–18 (1988)
U. Balachandran, N.G. Eror, Electrical conductivity in Ta2O5. Mater. Res. Bull. 17, 151–160 (1982)
A.E. Mchale, H.L. Tuller, Defects and charge transport in stabilized α-Ta2O5. Radiat. Effects. 75(1–4), 267–281 (1983)
A.E. McHale, H.L. Tuller, New tantala-based solid oxide electrolytes. Solid State Ionics 5, 515–518 (1981)
K.C. Anjaneyaa, J. Manjanna et al., Citrate-complexation synthesized Ce0.85Gd0.15O2−δ (GDC15) as solid electrolyte for intermediate temperature SOFC. Phys. B 447, 51–55 (2014)
X. Wang, T. Liu, C. Wang et al., Crystalline structure, microstructure and electrical characterizations of FeO1.5 doped YSZ. Ceram. Int. 43(12), 9577–9581 (2017)
H. Yoshida et al., Investigation of the relationship between the ionic conductivity and the local structures of singly and doubly doped ceria compounds using EXAFS measurement. Solid State Ionics 140(3–4), 191–199 (2001)
N.C. Stephenson, R.S. Roth, The crystal structure of the high temperature form of Ta2O5. J. Solid State Chem. 3, 145–153 (1971)
H. Inaba, Ceria-based solid electrolytes. Solid State Ionics 83(1–2), 1–16 (1996)
Y.W.C. Peng, K. Jiang et al., Study on the structure change and oxygen vacation shift for Ce1-xSmxO2−δ solid solution. J. Alloy Compd. 349, 273–278 (2003)
S.K. Tadokoro, E.N.S. Muccillo, Effect of Y and Dy co-doping on electrical conductivity of ceria ceramics. J. Eur. Ceram. Soc. 27(13–15), 4261–4264 (2007)
Y. Wang, Y.J. Jiang, Composition dependence and dielectric properties of (Ta2O5)1–x(TiO2)x polycrystalline ceramics. Mater. Sci. Eng. B 99(1–3), 221–225 (2003)
T. Damart, E. Coillet, A. Tanguy et al., Numerical study of the structural and vibrational properties of amorphous Ta2O5 and TiO2-doped Ta2O5. J. Appl. Phys. 119(17), 175106 (2016)
S. Tiwari, N. Khatun, N. Patra et al., Role of oxygen vacancies in Co/Ni substituted CeO2: a comparative study. Ceram. Int. 45(3), 3823–3832 (2019)
K. Venkataramana, C. Madhuri, C. Madhusudan et al., Investigation on La3+ and Dy3+ co-doped ceria ceramics with an optimized average atomic number of dopants for electrolytes in IT-SOFCs. Ceram. Int. 44(6), 6300–6310 (2018)
N. Kemnade, C.J. Shearer et al., Non-destructive functionalisation for atomic layer deposition of metal oxides on carbon nanotubes: effect of linking agents and defects. Nanoscale 7(7), 3028–3034 (2015)
X. Lu, Y. Zeng et al., Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 26(19), 3148–3155 (2014)
Yu Xin, W. Li, Z. Li et al., Defect engineered Ta2O5 nanorod: one-pot synthesis, visible-light driven hydrogen generation and mechanism. Appl. Catal. B 217, 48–56 (2017)
Y. Cheng, Y. Mao, B. Yuan et al., Enhanced negative thermal expansion and optical absorption of In0.6(HfMg)0.7Mo3O12 with oxygen vacancies. Phys. Lett. A. 381(27), 2195–2199 (2017)
D. Kuscer, I. Bantan, M. Hrovat et al., The microstructure, coefficient of thermal expansion and flexural strength of cordierite ceramics prepared from alumina with different particle sizes. J. Eur. Ceram. Soc. 37(2), 739–746 (2017)
Acknowledgements
The authors gratefully acknowledge the financial support of Hainan Provincial Natural Science Foundation of China (519MS022), the Key Scientific & Technological Project of Hainan Province (ZDKJ2017011), Special funds for guiding local scientific and technological development by China government (ZY2019HN0904), National Key Research and Development Program of China (2016YFC0700804), and Natural Science Foundation of China (51562008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, S., Zhang, J., Yang, S. et al. Effects of Cr/Ti co-doping on the electrical and thermal properties of tantalum-based electrolyte materials for solid oxide fuel cells. J Mater Sci: Mater Electron 31, 17307–17319 (2020). https://doi.org/10.1007/s10854-020-04286-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04286-7