Abstract
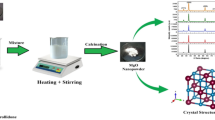
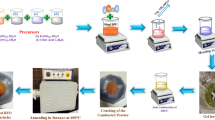
Nanostructured Co2O3 spinel is synthesized via simple co-precipitation method. The Co2O3 materials are analytically investigated with different techniques. However, the spinel type of Co2O3 nanoparticles showed attractive features for microwave and biomedical applications. X-ray diffraction (XRD) analysis indicates the growth of good crystalline Co2O3 nanoparticles with a cubic type. Field Emission Scanning Electron Microscope (FE-SEM) images of Co2O3 nanoparticles reveal spherical particles. In general, the magnetization of spinel Co2O3 nanoparticles demonstrates ferromagnetic order at low strength of the magnetic field. All the theoretical parameters of the metal oxide composite are done by utilizing DFT/B3LYP/LANL2DZ level of theory. The enhanced bond parameters, for example, bond lengths and bond angles, are determined utilizing same level of basis set. The non-linear optical (NLO) property of the title compound is assessed utilizing first appeal hyperpolarizability count. HOMO–LUMO investigation, the charge move ensues within the molecule. Moreover, Molecular electrostatic potential (MEP) and Mulliken atomic charges are likewise determined in detail.










Similar content being viewed by others
Change history
30 November 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10854-022-09389-x
References
M. Benelmekki, Nanomaterials: the original product of nanotechnology (Morgan & Claypool Publishers, 2019)
S. Mourdikoudis, R.M. Pallares, N.T. Thanh, Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 10(27), 12871–12934 (2018)
J. Jeevanandam, A. Barhoum, Y.S. Chan, A. Dufresne, M.K. Danquah, Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 9(1), 1050–1074 (2018)
K.J. Klabunde, R.M. Richards, Nanoscale Materials in Chemistry (Wiley, Hoboken, 2009)
M. Fernández-García, J.A. Rodriguez, Metal oxide nanoparticles. Encycl. Inorg. Bioinorg. Chem. (2011). https://doi.org/10.1002/9781119951438.eibc0331
J. Schmidt, M.R. Marques, S. Botti, M.A. Marques, Recent advances and applications of machine learning in solid-state materials science. NPJ Comput. Mater. 5(1), 1–36 (2019)
P.F. García, M. Brammen, M. Wolf, S. Reinlein, M.F. Von Roman, S. Berensmeier, High-gradient magnetic separation for technical scale protein recovery using low cost magnetic nanoparticles. Sep. Purif. Technol. 150, 29–36 (2015)
Y. Hou, H. Kondoh, M. Shimojo, T. Kogure, T. Ohta, High-yield preparation of uniform cobalt hydroxide and oxide nanoplatelets and their characterization. J. Phys. Chem. B 109(41), 19094–19098 (2005)
X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li, J. Gong, Enhanced surface reaction kinetics and charge separation of p–n heterojunction Co3O4/BiVO4 photoanodes. J. Am. Chem. Soc. 137(26), 8356–8359 (2015)
A.M. Cao, J.S. Hu, H.P. Liang, W.G. Song, L.J. Wan, X.L. He, X.G. Gao, S.H. Xia, Hierarchically structured cobalt oxide (Co3O4): the morphology control and its potential in sensors. J. Phys. Chem. B 110(32), 15858–15863 (2006)
J. Chen, X. Wu, A. Selloni, Electronic structure and bonding properties of cobalt oxide in the spinel structure. Phys. Rev. B 83(24), 245204 (2011)
A.S. Zola, R.U. Ribeiro, J.M.C. Bueno, D. Zanchet, P.A. Arroyo, Cobalt nanoparticles prepared by three different methods. J. Exp. Nanosci. 9(4), 398–405 (2012)
J. Morris, J. Willis, EPA Whitepaper on Nanotechnology, (2007)
M. Salavati-Niasari, F. Davar, M. Mazaheri, M. Shaterian, Preparation of cobalt nanoparticles from [bis (salicylidene) cobalt (II)]–oleylamine complex by thermal decomposition. J. Magn. Magn. Mater. 320(3–4), 575–578 (2008)
M. Mauro, M. Crosera, M. Pelin, C. Florio, F. Bellomo, G. Adami, P. Apostoli, G. De Palma, M. Bovenzi, M. Campanini, F.L. Filon, Cobalt oxide nanoparticles: behavior towards intact and impaired human skin and keratinocytes toxicity. Int. J. Environ. Res. Public Health 12(7), 8263–8280 (2015)
S.L. Sharifi, H.R. Shakur, A. Mirzaei, M.H. Hosseini, Characterization of cobalt oxide Co3O4 nanoparticles prepared by various methods: effect of calcination temperatures on size, dimension and catalytic decomposition of hydrogen peroxide. Int. J. Nanosci. Nanotechnol. 9(1), 51–58 (2013)
S.M. Ansari, R.D. Bhor, K.R. Pai, D. Sen, S. Mazumder, K. Ghosh, Y.D. Kolekar, C.V. Ramana, Cobalt nanoparticles for biomedical applications: facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl. Surf. Sci. 414, 171–187 (2017)
N. Izu, I. Matsubara, T. Uchida, T. Itoh, W. Shin, Synthesis of spherical cobalt oxide nanoparticles by a polyol method. J. Ceram. Soc. Jpn. 125(9), 701–704 (2017)
S. Farhadi, M. Javanmard, G. Nadri, Characterization of cobalt oxide nanoparticles prepared by the thermal decomposition. Acta Chim. Slov. 63(2), 335–343 (2016)
K. Sun, J. Wang, Y. Yang, Y. Li, Z. Yu, Z. Lan, X. Jiang, R. Guo, C. Wu, Influence of Ta2O5–Co2O3 co-doping on the magnetic property of NiMgCuZn ferrites. Physica B 476, 122–128 (2015)
K.P. Latha, C. Prema, S.M. Sundar, Synthesis and characterization of cobalt oxide nanoparticles. J. Nanosci. Technol. 4(5), 475–477 (2018)
Y. Guo, X. Jian, L. Zhang, C. Mu, L. Yin, J. Xie, N. Mahmood, S. Dou, R. Che, L. Deng, Plasma-induced FeSiAl@ Al2O3@ SiO2 core–shell structure for exceptional microwave absorption and anti-oxidation at high temperature. Chem. Eng. J. 384, 123371 (2020)
M.J. Frisch et al., Gaussian, Inc., Pittsburgh PA, (2009)
A.D. Becke, Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 96(3), 2155–2160 (1992)
C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37(2), 785 (1988)
P.J. Stephens, F.J. Devlin, C.F. Chabalowski, M.J. Frisch, Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98(45), 11623–11627 (1994)
C. Andraud, T. Brotin, C. Garcia, F. Pelle, P. Goldner, B. Bigot, A. Collet, Theoretical and experimental investigations of the nonlinear optical properties of vanillin, polyenovanillin, and bisvanillin derivatives. J. Am. Chem. Soc. 116(5), 2094–2102 (1994)
M. Nakano, H. Fujita, M. Takahata, K. Yamaguchi, Theoretical study on second hyperpolarizabilities of phenylacetylene dendrimer: toward an understanding of structure—property relation in NLO responses of fractal antenna dendrimers. J. Am. Chem. Soc. 124(32), 9648–9655 (2002)
V.M. Geskin, C. Lambert, J.L. Brédas, Origin of high second-and third-order nonlinear optical response in ammonio/borato diphenylpolyene zwitterions: the remarkable role of polarized aromatic groups. J. Am. Chem. Soc. 125(50), 15651–15658 (2003)
N.B. Colthup, L.H. Daly, S.E. Wiberley, Introduction to Infrared to and Raman Spectroscopy (Academic Press, New York, 1990)
I. Fleming, Frontier Orbitals and Organic Chemical Reactions (Wiley , New York, 1976)
N.M. O’boyle, A.L. Tenderholt, K.M. Langner, Cclib: a library for package-independent computational chemistry algorithms. J. Comput. Chem. 29(5), 839–845 (2008)
E. Scrocco, J. Tomasi, Electronic molecular structure, reactivity and intermolecular forces: an euristic interpretation by means of electrostatic molecular potentials. Adv. Quantum Chem. 11, 115–193 (1978)
F.J. Luque, J.M. López, M. Orozco, Perspective on “electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects.” Theoret. Chem. Acc. 103(3–4), 343–345 (2000)
N. Okulik, A.H. Jubert, Theoretical analysis of the reactive sites of non-steroidal anti-inflammatory drugs. Internet Electron. J. Mol. Design 4(1), 17–30 (2005)
R.S. Mulliken, Electronic population analysis on LCAO–MO molecular wave functions. IV. Bonding and antibonding in LCAO and valence‐bond theories. J. Chem. Phys. 23(12), 2343–2346 (1955)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajeevgandhi, C., Sathiyamurthy, K., Guganathan, L. et al. RETRACTED ARTICLE: Experimental and theoretical investigations on the spinel structure of Co2O3 nanoparticles synthesized via simple co-precipitation method. J Mater Sci: Mater Electron 31, 16769–16779 (2020). https://doi.org/10.1007/s10854-020-04232-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04232-7