Abstract
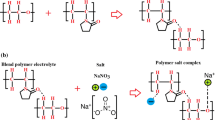
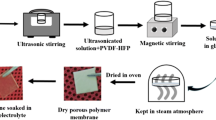
In the present study, electrochemical impedance analysis in terms of electrical conductivity, dielectric permittivity, and electrical modulus has been carried out of prepared sodium ion-conducting nanocomposite gel polymer electrolyte. To study ion conduction behavior, frequency-dependent AC conductivity has also been analyzed. Dielectric constant (ε′) and dielectric loss (ε′′) as a function of frequency with different nanofiller SiO2 concentrations as well as at different temperatures ranging from 303 to 333 K have been discussed. The low-frequency region showed high values of dielectric constant due to polarization at the electrode–electrolyte interface. Frequency-dependent real (M′) and imaginary part (M′′) of modulus reveal large capacitance associated with it at lower frequency whereas dispersion (conductivity relaxation) at a higher frequency. The tangent loss (tan δ) of the electrolyte systems has been determined for different frequencies and concentrations of fumed silica nanoparticles. The high conducting nanocomposite gel polymer membrane exhibited an electrochemical stability window of ≈ 3.3 V which is sufficient to apply this material as a separator for electrochemical device application. The conductivity, dielectric, modulus, and electrochemical stability studies reveal that sodium ion-conducting nanocomposite gel polymer electrolytes offer good electrochemical properties and are suitable for application in any electrochemical/power conversion device. The optimized flexible nanocomposite gel polymer electrolyte films have been used in a prototype sodium battery, which shows a stable open-circuit potential of ~ 2.1 V and a significant first specific discharge capacity of ~ 500 mAh g−1 at a drain current of 14 mA g−1.












Similar content being viewed by others
References
J.B. Goodenough, K.-S. Park, The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135(4), 1167–1176 (2013)
O.V. Lonchakova, O.A. Semenikhin, M.V. Zakharkin, E.A. Karpushkin, V.G. Sergeyev, E.V. Antipov, Efficient gel-polymer electrolyte for sodium-ion batteries based on poly(acrylonitrile-co-methyl acrylate). Electrochim. Acta 334, 135512 (2020)
D. Kumar, D.K. Kanchan, S.B. Kuhar, Room temperature sodium-sulfur batteries as emerging energy source. J. Energy Storage 18, 133–148 (2018)
R. Deivanayagam, B.J. Ingram, R.S. Yassar, Progress in development of electrolytes for magnesium batteries. Energy Storage Mater. 21, 136–153 (2019)
C. Maheshwaran, D.K. Kanchan, K. Gohel, K. Mishra, D. Kumar, Effect of Mg(CF3SO3)2 concentration on structural and electrochemical properties of ionic liquid incorporated polymer electrolyte membranes. J. Solid State Electrochem. 24, 655–665 (2020). https://doi.org/10.1007/s10008-020-04507-3
X. Zhang, D. Yang, X. Rui, Y. Yu, S. Huang, Advanced cathodes for potassium-ion battery. Curr. Opin. Electrochem. 18, 24–30 (2019)
M. Xu, D.G. Ivey, Z. Xie, W. Qu, Rechargeable Zn-air batteries: progress in electrolyte development and cell configuration advancement. J. Power Sources 283, 358–371 (2015)
A.R. Despić, Design characteristics of an aluminium-air battery with consumable wedge anodes. J. Appl. Electrochem. 15, 191–200 (1985)
A. Chandrasekhar, V. Vivekananthan, S.-J. Kim, A fully packed spheroidal hybrid generator for water wave energy harvesting and self-powered position tracking. Nano Energy 69, 104439 (2020)
A. Sukumaran, V. Vivekananthan, V. Mohan, Z.C. Alex, A. Chandrasekhar, S.-J. Kim, Triboelectric nanogenerators from reused plastic: an approach for vehicle security alarming and tire motion monitoring in rover. Appl. Mater. Today 19, 100625 (2020)
A. Chandrasekhar, V. Vivekananthan, G. Khandelwal, S.-J. Kim, Sustainable human-machine interactive triboelectric nanogenerator toward a smart computer mouse. ACS Sustain. Chem. Eng. 7(7), 7177–7182 (2019)
A. Chandrasekhar, N.R. Alluri, M.S.P. Sudhakaran, Y.S. Mokd, S.-J. Kim, A smart mobile pouch as a biomechanical energy harvester towards self-powered smart wireless power transfer applications. Nanoscale 9, 9818–9824 (2017)
A. Chandrasekhar, N.R. Alluri, V. Vivekananthan, Y. Purusothamana, S.-J. Kim, A sustainable freestanding biomechanical energy harvesting smart backpack as a portable-wearable power source. J. Mater. Chem. C 5, 1488–1493 (2017)
A. Ponrouch, D. Monti, A. Boschin, B. Steen, P. Johansson, M.R. Palacín, Non-aqueous electrolytes for sodium-ion batteries. J. Mater. Chem. A 3, 22–42 (2015)
A. Kumar, S.A. Hashmi, Ion transport and ion–filler-polymer interaction in poly(methyl methacrylate)-based, sodium ion conducting, gel polymer electrolytes dispersed with silica nanoparticles. J. Power Sources 195, 5101–5108 (2010)
M. Jahn, M. Sedlaříková, J. Vondrák, L. Pařízek, PMMA-based electrolytes for li-ion batteries. ECS Trans. 74(1), 159–164 (2016)
M. Zhu, J. Wu, Y. Wang, M. Song, L. Long, S.H. Siyal, X. Yang, G. Sui, Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 37, 126–142 (2019)
K. Mishra, T. Arif, R. Kumar, D. Kumar, Effect of Al2O3 nanoparticles on ionic conductivity of PVdF-HFP/PMMA blend-based Na+-ion conducting nanocomposite gel polymer electrolyte. J. Solid State Electrochem. 23(8), 2401–2409 (2019)
D.J. Kim, M.J. Jo, S.Y. Nam, A review of polymer–nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 21, 36–52 (2015)
H. Che, S. Chen, Y. Xie, H. Wang, K. Amine, X.-Z. Liao, Z.-F. Ma, Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 10, 1075–1101 (2017)
L.G. Chagas, S. Jeong, I. Hasa, S. Passerini, Ionic liquid-based electrolytes for sodium-ion batteries: tuning properties to enhance the electrochemical performance of manganese-based layered oxide cathode. ACS Appl. Mater. Interfaces 11(25), 22278–22289 (2019)
D. Kumar, Effect of organic solvent addition on electrochemical properties of ionic liquid-based Na+ conducting gel electrolytes. Solid State Ionics 318, 65–70 (2018)
C.-H. Dustmann, Advances in ZEBRA batteries. J. Power Sources 127, 85–92 (2004)
R. Wang, H. Mo, S. Li, Y. Gong, B. He, H. Wang, Influence of Conductive additives on the stability of red phosphorus carbon anodes for sodium-ion batteries. Sci. Rep. 9, 946 (2019). https://doi.org/10.1038/s41598-018-36797-z
W. Tian, L. Wang, K. Huo, X. He, Red phosphorus filled biomass carbon as high-capacity and long-life anode for sodium-ion batteries. J. Power Sources 430, 60–66 (2019)
Z. Li, H. Zhao, Recent developments of phosphorus-based anodes for sodium ion batteries. J. Mater. Chem. A 6, 24013 (2018)
H. Liu, S. Zhang, Q. Zhu, B. Cao, P. Zhang, N. Sun, B. Xu, F. Wua, R. Chen, Fluffy carbon-coated red phosphorus as a highly stable and high-rate anode for lithium-ion batteries. J. Mater. Chem. A7, 11205–11213 (2019)
S. Sharma, D. Pathak, N. Dhiman, R. Kumarand, M. Kumar, FTIR, thermal and ionic conductivity studies of nanocomposite polymer electrolytes. Surf. Innov. 7(1), 51–58 (2019)
K.W. Chew, K.W. Tan, The effects of ceramic fillers on PMMA-based polymer electrolyte salted with lithium triflate, LiCF3SO3. Int. J. Electrochem. Sci. 6, 5792–5801 (2011)
B.A. Boukamp, A Linear Kronig−Kramers transform test for immittance data validation. J. Electrochem. Soc. 142, 1885–1894 (1995)
N.K. Singh, M.L. Verma, M. Minakshi, PEO nanocomposite polymer electrolyte for solid state symmetric capacitors. Bull. Mater. Sci. 38, 1577–1588 (2015)
K. Gohel, D.K. Kanchan, Ionic conductivity and relaxation studies in PVDF-HFP:PMMA-based gel polymer blend electrolyte with LiClO4 salt. J. Adv. Dielectrics 08(01), 1850005 (2018)
F. Croce, B. Scrosati, G. Mariotto, Electrochemical and spectroscopic study of the transport properties of composite polymer electrolytes. J. Chem. Mater. 4, 1134–1136 (1992)
S.B. Aziz, J.W. Thompson, M.F.Z. Kadir, H.M. Ahmed, A conceptual review on polymer electrolytes and ion transport models. J. Sci. 3, 1–17 (2018)
K. Gohel, D.K. Kanchan, Effect of PC: DEC plasticizers on structural and electrical properties of PVDF–HFP:PMMA based gel polymer electrolyte system. J. Mater. Sci. 30, 12260–12268 (2019)
S. Choudhary, R.J. Sengwa, Effects of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes. Electrochim. Acta 247, 924–941 (2017)
W. Wang, P. Alexandridis, Composite polymer electrolytes: nanoparticles affect structure and properties. Polymers 8, 1–36 (2016)
M. Hema, P. Tamilselvi, Lithium ion conducting PVA:PVdF polymer electrolytes doped with nano SiO2 and TiO2 filler. J. Phys. Chem. Solids 97–98, 42–48 (2016)
P. Lun, P. Liu, H. Lin, Z. Dai, Z. Zhang, D. Chen, Ionic conductivity promotion of polymer membranes with oxygen-ion conducting nanowires for rechargeable lithium batteries. J. Membr. Sci. 580, 92–100 (2019)
A. Bhardwaj, A. Kumar, H. Bae, C.-J. Park, S.-J. Song, Surface decorated spinel-oxide electrodes for mixed-potential ammonia sensor: performance and DRT analysis. J. Hazard. Mater. 396, 122601 (2020)
M.S. Munde, D.Z. Gao, A.L. Shluger, Diffusion and aggregation of oxygen vacancies in amorphous silica. J. Phys. 29, 245701 (2017)
A. Karmakar, A. Ghosh, Dielectric permittivity and electric modulus of polyethylene oxide (PEO)-LiClO4 composite electrolytes. Curr. Appl. Phys. 12, 539–543 (2012)
K.S. Hemalatha, G. Sriprakash, M.V.N.A. Prasad, R. Damle, K. Rukmani, Temperature dependent dielectric and conductivity studies of polyvinyl alcohol-ZnO nanocomposite films by impedance spectroscopy. J. Appl. Phys. 118, 154103–154116 (2015)
A. Kyritsis, P. Pissis, J. Grammatikakis, Dielectric relaxation spectroscopy in poly(hydroxyethylene acrylates)/water hydrogels. J. Poly. Sci. Part B Poly. Phys. 33, 1737–1750 (1995)
T. Regu, C. Ambika, K. Karuppasamy, H. Rajan, D. Vikraman, J.-H. Jeon, H.-S. Kim, T.A.B. Raj, Proton transport and dielectric properties of high molecular weight polyvinylpyrrolidone (PVPK90) based solid polymer electrolytes for portable electrochemical devices. J. Mater. Sci. 30, 11735–11747 (2019)
P. Sharma, D.K. Kanchan, A comparison of effect of PEG and EC plasticizers on relaxation dynamics of PEO-PMMA-AgNO3 polymer blend. Ionics 19, 1285–1290 (2013)
D.K. Pradhan, R.N.P. Choudhary, B.K. Samantaray, Studies of dielectric and electrical properties of plasticized polymer nano composite electrolytes. J. Mater. Chem. Phys. 115, 557–561 (2009)
J.Y. Hwang, S.T. Myung, Y.K. Sun, Sodium-ion batteries: present and future. Chem. Soc. Rev. 46, 3529–3614 (2017)
D. Kumar, D.K. Kanchan, S. Kumar, K. Mishra, Recent trends on tailoring cathodes for room-temperature Na-S batteries. Mater. Sci. Energy Technol. 2, 117–129 (2019)
V. Kumar, Y. Wang, A.Y.S. Eng, M.-F. Ng, Z.W. She, A biphasic interphase design enabling high performance in room temperature sodium-sulfur batteries. Cell Rep. Phys. Sci. (2020). https://doi.org/10.1016/j.xcrp.2020.100044
V. Kumar, A.Y. Sheng Eng, Y. Wang, D.-T. Nguyen, M.-F. Ng, Z.W. Seh, An artificial metal-alloy interphase for high-rate and long-life sodium–sulfur batteries. Energy Storage Mater. 15, 20 (2020). https://doi.org/10.1016/j.ensm.2020.03.027
Acknowledgements
Deepak Kumar thanks and acknowledges “The M.S. University of Baroda,” Vadodara, Gujarat, India. He thanks and acknowledges Dr. S.A. Hashmi, University of Delhi, Delhi, India, for constant motivation and support. The encouragement received from Electronics and Mechanical Engineering School (Affiliated to Gujarat Technological University), Corps of Electronics and Mechanical Engineers, Ministry of Defence, Government of India, is highly acknowledged. Part of this research work was performed at University of Delhi, Delhi, India. Kuldeep Mishra acknowledges the funding (File No. YSS/2015/001234) from Science and Engineering Research Board (SERB) New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicting interest in any capacity, competing, or financial.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, D., Gohel, K., Kanchan, D.K. et al. Dielectrics and battery studies on flexible nanocomposite gel polymer electrolyte membranes for sodium batteries. J Mater Sci: Mater Electron 31, 13249–13260 (2020). https://doi.org/10.1007/s10854-020-03877-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03877-8