Abstract
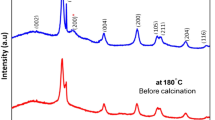
Well-crystalline NiO–CeO2 nanocomposites have been fabricated by ignition method and investigated by X-ray diffraction, Fourier Transform Infrared, UV–Vis diffuse reflectance spectroscopy, Thermal gravimetric analysis, BET surface area, and transmission electron microscopy. The detailed characterizations disclosed that the pre-calcine (700 °C) nanocomposite (NCC) has two pure phases: cubic fluorite phase (CeO2) and cubic face-centered phase (NiO). Finally, the pre-calcine NCC nanocomposite was applied as electron intermediators for the electrochemical sensing of 4-nitrophenol (4-NP). Compared with as-grown modified electrode (NCG/GCE), pre-calcine electrode (NCC/GCE) exhibited more excellent conductivity and better electrocatalytic mediator for 4-NP. It was found that the NCC/GCE sensor displayed diffusion-controlled kinetics and excellent sensitivity (3.68 AμM−1 cm−2). The reduction current is directly proportional to the 4-NP concentration, ranging from 1 to 20 μM with lower detection limit of 2.48 μM.











Similar content being viewed by others
References
A.L. Buikema, M.J. McGimres, J. Cairns, Phenolics in aquatic ecosystems: a selected review of recent literature. Mar. Environ. Res. 2, 87–181 (1979)
C. Schummer, C. Groff, J.A. Chami, F. Jaber, M. Millet, Analysis of phenols and nitrophenols in rainwater collected simultaneously on an urban and rural site in east of France. Sci. Total Environ. 407, 5637–5643 (2009)
P. Wang, J. Xiao, A. Liao, P. Li, M. Guo, Y. Xia, Electrochemical determination of 4-nitrophenol using uniform nanoparticle film electrode of glass carbon fabricated facilely by square wave potential pulses. Electrochim. Acta 176, 448–455 (2015)
X.M. Xu, Z. Liu, X. Zhang, S. Duan, S. Xu, C.L. Zhou, Cyclodextrin functionalized mesoporous silica for electrochemical selective sensor: simultaneous determination of nitrophenol isomers. Electrochim. Acta 58, 142–149 (2011)
S.S. Li, D. Du, J. Huang, H.Y. Tu, Y.Q. Yang, A.D. Zhang, One-step electrode-position of a molecularly imprinting chitosan/phenyltrimethoxysilane/AuNPshybrid film and its application in the selective determination of p-nitrophenol. Analyst 138, 2761–2768 (2013)
J.A. Padilla-Sanchez, P. Plaza-Bolanos, R. Romero-Gonzalez, N. Barco-Bonilla, J.L. Martin ez-Vidal, A. Garrido-Frenich, Simultaneous analysis of chlorophenols, alkyl phenols, nitrophenols and cresols in waste water effluents, using solid phase extraction and further determination by gas chromatography-tandem mass spectro metry. Talanta 85, 2397–2404 (2011)
R.J. Chung, M.I. Leong, S.D. Huang, Determination of nitrophenols using ultra- high pressure liquid chromatography and a new manual shaking-enhanced, ultra sound-assisted emulsification microextraction method based on solidification of a floating organic droplet. J. Chromatogr. A 1246, 55–61 (2012)
M.C. Alcudia-Leon, R. Lucena, S. Cardenas, M. Valcarcel, Determination of phenols in waters by stir membrane liquid-liquid-liquid microextraction coupled to liquid chromatography with ultraviolet detection. J Chromatogr. A 1218(2176–21), 81 (2011)
F. Bagheban-Shahri, A. Niazi, A. Akrami, Simultaneous spectrophotometric determination of nitrophenol isomers in environmental samples using first derivative of the density ratio spectra. J. Chem. Health Risks 2, 21–28 (2012)
X.F. Guo, Z.H. Wang, S.P. Zhou, The separation and determination of nitrophenol isomers by high-performance capillary zone electrophoresis. Talanta 64, 135–139 (2004)
M. Manera, M. Miró, J.M. Estela, V. Cerdà, M.A. Segundo, J.L.F.C. Lima, Flow-through solid-phase reflectometric method for simultaneous multiresidue determina- tion of nitrophenol derivatives. Anal. Chim. Acta 600, 155–163 (2007)
S. Tingry, C. Innocent, S. Touil, A. Deratani, P. Seta, Carbon paste biosensor for phenol detection of impregnated tissue: modification of selectivity by using bcyclo dextrin-containing PVA membrane. Mater. Sci. Eng. C 26, 222–226 (2006)
J. Li, D. Kuang, Y. Feng, F. Zhang, Z. Xu, M. Liu, A graphene oxide-based electro chemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 201–202, 250–259 (2012)
Y. Xu, Y. Wang, Y. Ding, L. Luo, X. Liu, Y. Zhang, Determination of p-nitro phenol on carbon paste electrode modified with a nanoscaled compound oxide Mg (Ni) FeO. J. Appl. Electrochem. 43, 679–687 (2013)
Y.L. Yang, B. Unnikrishnan, S.M. Chen, Amperometric determination of 4- nitro phenol at multi-walled carbon nanotube-poly(diphenylamine) composite modified glassy carbon electrode. Int. J. Electrochem. Sci. 6, 3902–3912 (2011)
H. Yin, Q. Ma, Y. Zhou, S. Ai, L. Zhu, Electrochemical behavior and voltammetric determination of 4-aminophenol based on graphene–chitosan composite film modified glassy carbon electrode. Electrochim. Acta 55, 7102–7108 (2010)
L.Q. Luo, X.L. Zou, Y.P. Ding, Q.S. Wu, Derivative voltammetric direct simultane- ous determination of nitrophenol isomers at a carbon nanotube modified electrode. Sens. Actuators B 135, 61–65 (2008)
L. Chu, L. Han, X. Zhang, Electrochemical simultaneous determination of nitro phenol isomers at nano-gold modified glassy carbon electrode. J. Appl. Electrochem. 41(6), 687–694 (2011)
I.G. Casella, M. Contursi, The electrochemical reduction of nitrophenols on silver globular particles electrodeposited under pulsed potential conditions. J Electron. Chem. Soc. 154, D697–D702 (2007)
W. Sun, M.X. Yang, Q. Jiang, K. Jiao, Direct electrocatalytic reduction of p-nitro phenol at room temperature ionic liquid modified electrode. Chin. Chem. Lett. 19, 1156–1158 (2008)
X. Xie, Y. Li, Z. Liu, M. Haruta, W. Shen, Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458(7239), 746–749 (2009)
I. Celardo, J.Z. Pedersen, E. Traversa, L. Ghibelli, Pharmacological potential of cerium oxide nanoparticles. Nanoscale 3, 1411–1420 (2011)
X.Q. Liu, J. Iocozzia, Y. Wang, X. Cui, Y.H. Chen, S.Q. Zhao, Z. Li, Z.Q. Lin, Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 10, 402–434 (2017)
N.R. Elezovic, B.M. Babic, V.R. Radmilovic, S.L. Gojkovic, N.V. Krstajic, L.M. Vracar, Pt/C doped by MoOx as the electrocatalyst for oxygen reduction and methanol oxidation. J. Power Sources 175, 250–255 (2008)
Y. Bai, J. Wu, J. Xi, J. Wang, W. Zhu, L. Chen, X. Qiu, Electrochemical oxidation of ethanol on Pt–ZrO2/C catalyst. Electrochem. Commun. 7, 1087–1090 (2005)
L. Feng, J. Yang, Y. Hu, J. Zhu, C. Liu, W. Xing, Electrocatalytic properties of Pd CeOx/C anodic catalyst for formic acid electrooxidation. Int. J. Hydrog Energy 37, 4812–4818 (2012)
C. Zhang, F. Meng, L. Wang, M. Zhang, Z. Ding, Morphology-selective synthesis method of gear-like CeO2 microstructures and their optical properties. Mater. Lett. 130, 202–205 (2014)
C.R. Li, Q.T. Sun, N.P. Lu, B.Y. Chen, W.J. Dong, A facile route for the fabrication of CeO2 nanosheets via controlling the morphology of CeOHCO3 precursors. J. Cryst. Growth 343, 95–100 (2012)
R. Rao, M. Yang, Q. Ling, Q. Zhang, H. Liu, A. Zhang, W. Chen, Mesoporous CeO2 nanobelts synthesized by a facile hydrothermal route via controlling cationic type and concentration of alkali. Microporous Mesoporous Mater. 169, 81–87 (2013)
F. Niu, D. Zhang, L. Shi, X. He, H. Li, H. Mai, T. Yan, Facile synthesis, characteri- zation and low-temperature catalytic performance of Au/CeO2 nanorods. Mater. Lett. 63, 2132–2135 (2009)
K. Singh, A.A. Ibrahim, A. Umar, A. Kumar, G.R. Chaudhary, S. Singh, S.K. Mehta, Synthesis of CeO2–ZnO nanoellipsoids as potential scaffold for the efficient detection of 4-nitrophenol. Sens. Actuators B 202, 1044–1050 (2014)
C.R. Michel, A.H.M. Preciado, CO sensor based on thick films of 3D hierarchical CeO2 architectures. Sens. Actuators B 197, 177–184 (2014)
N.F. Hamedani, A.R. Mahjoub, A.A. khodadadi, Y. Mortazavi, CeO2 doped ZnO flower-like nanostructure sensor selective to ethanol in presence of CO and CH4. Sens. Actuators B 169, 67–73 (2012)
S.K. Jha, C.N. Kumar, R.P. Raj, N.S. Jha, S. Mohan, Synthesis of 3D porous CeO2/reduced graphene oxide xerogel composite and low level detection of H2O2. Electrochim. Acta 120, 308–313 (2014)
A. Rahim, R.A. Hameed, M. Khalil, Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 134, 160–169 (2004)
C. Fan, D. Piron, A. Sleb, P. Paradis, Study of electrodeposited nickel molybdenum, nickel tungsten, cobalt molybdenum, and cobalt tungsten as hydrogen electr odes in alkaline water electrolysis. J. Electrochem. Soc. 141, 382–387 (1994)
M. Ranjbar, M.A. Taher, A. Sam, Mg-MOF-74 nanostructures: facile synthesis and characterization with aid of 2,6-pyridinedicarboxylic acid ammonium. J. Mater. Sci.: Mater. Electron. 27(2), 1449–1456 (2016)
M. Ranjbar, M.S. Niasari, S.M.H. Mashkani, K.V. Rao, Solvothermal synthesis and characterization of hollow sphere-like ZnS/ZnAl2S4 nanocomposites. J. Inorg. Organomet. Polym. Mater. 22(5), 1122–1127 (2012)
P. Rajaei, M. Ranjbar, Synthesis and characterization of zinc oxide nano structures by green capping agent and its photocatalytic degradation of methylene blue (MB). J. Mater. Sci.: Mater. Electron. 27(2), 1708–1712 (2016)
M. Ranjbar, M.A. Taher, A. Sam, NiO nanostructures: novel solvent-less solid-state synthesis, characterization and MB photocatalytic degradation. J. Mater. Sci.: Mater. Electron. 26(10), 8029–8034 (2015)
F. Sedighi, M.E. Zare, A.S. Nasab, M. Behpour, Synthesis and characterization of CuWO4 nanoparticle and CuWO4/NiO nanocomposite using co-precipitation method; application in photodegradation of organic dye in water. J. Mater. Sci.: Mater. Electron. 29(16), 13737–13745 (2018)
M.R. Nasrabadi, M. Behpour, A.S. Nasab, M.R. Jeddy, Nanocrystalline Ce-doped copper ferrite: synthesis, characterization, and its photocatalyst application. J. Mater. Sci.: Mater. Electron. 27(11), 11691–11697 (2016)
A.S. Nasab, S. Pourmasoud, F. Ahmadi, M. Wysokowski, T. Jesionowski, H. Ehrlich, M.R. Nasrabadi, Synthesis and characterization of MnWO4/TmVO4 ternary nano-hybrids by an ultrasonic method for enhanced photocatalytic activity in the degradation of organic dyes. Mater. Lett. 238, 159–162 (2019)
H. Naderi, H. Sobati, A.S. Nasab, M.R. Nasrabadi, M.E. Arani, M.R. Ganjali, H. Ehrlich, Synthesis and supercapacitor application of cerium tungstate nano structure. Chem. Select 4(10), 2862–2867 (2019)
S.M. Pourmortazavi, M.R. Nasrabadi, A.S. Nasab, M.S. Karimi, M.R. Ganjali, S. Mirsadeghi, Electrochemical synthesis of copper carbonates nano particles through experimental design and the subsequent thermal decomposition to copper oxide. Mater. Res. Express. 6(4), 045065 (2019)
A.S. Nasab, M.R. Nasrabadi, H.R. Naderi, V. Pourmohamadian, F. Ahmadi, M.R. Ganjali, H. Ehrlich, Sonochemical synthesis of terbium tungstate for develo ping high power supercapacitors with enhanced energy densities. Ultrason. Sonochem 45, 189–196 (2018)
D. Patil, N.Q. Dung, H. Jung, S.Y. Ahn, D.M. Jang, D. Kim, Enzymatic glucose bio sensor based on CeO2 nanorods synthesized by non-isothermal precipitation. Biosens. Bioelectron. 31, 176–181 (2012)
H. Yan, D. Zhang, J. Xu, Y. Lu, Y. Liu, K. Qiu, Y. Zhang, Y. Luo, Solution growth of NiO nanosheets supported on Ni foam as high performance electrodes for Super capacitors. Nanoscale Res. Lett. 9, 424 (2014)
R. Wahab, A. Umar, S. Dwivedi, K.J. Tomar, H.S. Shin, I.H. Hwang, ZnO nano particles: cytological effect on chick fibroblast cells and antimicrobial activities towards Escherichia coli and Bacillus subtilis. Sci. Adv. Mater. 5, 1571–1580 (2013)
L. Chen, L. Li, G. Li, Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 464, 532–536 (2008)
E. Kumar, P. Selvarajan, K. Balasubramanian, Preparation and studies of cerium dioxide (CeO2) nanoparticles by microwave-assisted solution method. Recent Res. Sci. Technol. 2, 37–41 (2010)
Y. Li, X. Liu, J. Li, Preparation and characterization of CeO2 doped ZnO nano-tubes fluorescent composite. J. Rare Earth 28, 571–575 (2010)
T. Ahmad, K.V. Ramanujachary, S.E. Lofland, A.K. Ganguli, Magnetic and electro chemical properties of nickel oxide nanoparticles obtained by the reverse-micellar route. Solid State Sci. 8(42), 5–430 (2006)
X.M. Ni, Q.B. Zhao, B.B. Li, J. Cheng, H.G. Zheng, Interconnected β-Ni(OH)2 sheets and their morphology-retained transformation into mesostructured Ni. Solid State Commun. 137, 585–588 (2006)
X. Li, J. Li, D. Huo, Z. Xiu, X. Sun, Facile synthesis under near-atmospheric conditions and physicochemical properties of hairy CeO2 nanocrystallines. J. Phys. Chem. C 113, 1806–1811 (2009)
S.J. Gregg, K.S.W. Sing, Adsorption, surface area and porosity (Academic Press, London, 1982)
S. Lowell, J.E. Shields, Powder surface area and porosity (Chapman and Hall, London, 1984)
G. Wang, Y. Bao, Y. Tian, J. Xia, D. Cao, Electrocatalytic activity of perovskite La1-xSrx MnO3 towards hydrogen peroxide reduction in alkaline medium. J. Power Sources 195, 6463–6467 (2010)
N.Q. Dung, D. Patil, H. Jung, J. Kim, D. Kim, NiO-decorated single-walled carbon nanotubes for high-performance nonenzymatic glucose sensing. Sens. Actuators B 183, 381–387 (2013)
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-218.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, N., Alam, M., Wahab, R. et al. Synthesis of NiO–CeO2 nanocomposite for electrochemical sensing of perilous 4-nitrophenol. J Mater Sci: Mater Electron 30, 17643–17653 (2019). https://doi.org/10.1007/s10854-019-02113-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02113-2