Abstract
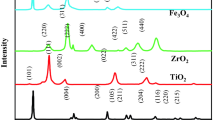
This work was aimed to prepare Fe doped TiO2 on an ionic liquid template so that nanoparticles (IL-Fe/TiO2(Ar)) could be formed to degrade acetaminophen (ACE) using the photocatalytic process. The synthesized nanoparticles were characterized using XRD, DRS, FTIR and FESEM–EDX. IL-Fe/TiO2(Ar) enhanced ACE photodegradation using the response surface methodology based on central composite design. The results evidenced that the presence of the 1-octadecyl-3-methylimidazolium bromide template led to development of nanocrystals with a uniform, small grain size. Moreover, the band gap energy calculated for IL-Fe/TiO2(Ar) was observed to be 1.6 eV. The ANOVA results of the photodegradation process revealed a second term model with an adjusted R2 equal to 0.996. The results also showed an ACE removal efficiency of 90.35% under optimal conditions (IL-Fe/TiO2(Ar) dosage = 0.63 g/L, pH 9 and UV irradiation time = 90.35 min). According to the findings, the pseudo first order model was introduced to study photodegradation kinetics. The findings also yielded insight into the most effective variables on degradation of ACE, which were time, IL-Fe/TiO2(Ar) and pH, respectively. Moreover, IL-Fe/TiO2(Ar) had high potential for the removal of ACE by photodegradation and proved to be an appropriate method to obtain optimum operating conditions.









Similar content being viewed by others
References
A. Gómez-Avilés, M. Peñas-Garzón, J. Bedia, J.J. Rodriguez, C. Belver, Chem. Eng. J. 358, 1574–1582 (2019)
M. Liu, R. Inde, M. Nishikawa, X. Qiu, D. Atarashi, E. Sakai, Y. Nosaka, K. Hashimoto, M. Miyauchi, ACS Nano 8, 7 (2014)
M. Malakootian, H. Mahdizadeh, A. Dehdarirad, M. Amiri Gharghani, J. Dispers. Sci. Technol. 40, 846–854 (2018)
Y. Ling, G. Liao, P. Xu, L. Li, Sep. Purif. Technol. 216, 1–8 (2019)
M. Malakootian, M. Pourshaban-Mazandarani, H. Hossaini, M.H. Ehrampoush, Process. Saf. Environ. 104, 334–345 (2016)
N. Muir, J.D. Nichols, M.R. Stillings, J. Sykes, Curr. Med. Res. Opin. 13, 9 (1997)
T. Lin, S. Yu, W. Chen, Chemosphere 152, 1–9 (2016)
A. Nasiri, F. Tamaddon, M.H. Mosslemin, M.A. Gharaghani, A. Asadipour, J. Mater. Sci.: Mater. Electron. 30, 9 (2019)
S.M. Peymani-Motlagh, A. Sobhani-Nasab, M. Rostami, H. Sobati, M. Eghbali-Arani, M. Fasihi-Ramandi, M.R. Ganjali, M. Rahimi-Nasrabadi, J. Mater. Sci.: Mater. Electron. 30, 7 (2019)
M. Malakootian, N. Olama, M. Malakootian, A. Nasiri, Int. J. Environ. Sci. Technol. (2018). https://doi.org/10.1007/s13762-018-1836-2
A.L. Ahmad, N.D. Zaulkiflee, A. Kusumastuti, M.M.H.S. Buddin, Ind. Eng. Chem. Res. 58, 2 (2019)
V.H.N. Phong, T. Koottatep, S.K. Chapagain, A. Panuvatvanich, C. Polprasert, K.-H. Ahn, Environ. Eng. Res. 21, 2 (2016)
C.-C. Su, C.A. Cada, M.L.P. Dalida, M.-C. Lu, Sep. Purif. Technol. 120, 43–51 (2013)
E. Villaroel, J. Silva-Agredo, C. Petrier, G. Taborda, R.A. Torres-Palma, Ultrason. Sonochem. 21, 5 (2014)
R. Andreozzi, V. Caprio, R. Marotta, D. Vogna, Water Res. 37, 5 (2003)
A. Aguinaco, F.J. Beltrán, J.F. García-Araya, A. Oropesa, Chem. Eng. J. 189–190, 275–282 (2012)
C. Maria Magdalane, K. Kaviyarasu, N. Matinise, N. Mayedwa, N. Mongwaketsi, D. Letsholathebe, G.T. Mola, N. AbdullahAl-Dhabi, M.V. Arasu, M. Henini, J. Kennedy, M. Maaza, B. Jeyaraj, S. Afr. J. Chem. Eng. 26, 49–60 (2018)
A. Sobhani-Nasab, M. Rangraz-Jeddy, A. Avanes, M. Salavati-Niasari, J. Mater. Sci.: Mater. Electron. 26, 12 (2015)
A. Sobhani-Nasab, M. Behpour, M. Rahimi-Nasrabadi, F. Ahmadi, S. Pourmasoud, J. Mater. Sci.: Mater. Electron. 30, 6 (2019)
N. Seifvand, E. Kowsari, Ind. Eng. Chem. Res. 55, 40 (2016)
M. Salavati-Niasari, F. Soofivand, A. Sobhani-Nasab, M. Shakouri-Arani, M. Hamadanian, S. Bagheri, J. Mater. Sci.: Mater. Electron. 28, 20 (2017)
A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, M. Salavati-Niasari, H. Taqriri, S. Bagheri, K. Saberyan, J. Mater. Sci.: Mater. Electron. 26, 8 (2015)
X. Wang, Y. Tang, M.-Y. Leiw, T.-T. Lim, Appl. Catal. A 409–410, 257–266 (2011)
S.M. Hosseinpour-Mashkani, A. Sobhani-Nasab, J. Mater. Sci.: Mater. Electron. 28, 5 (2017)
Y. Lai, W. Liu, J. Fang, F. Qin, M. Wang, F. Yu, K. Zhang, RSC Adv. 5, 114 (2015)
C.J. Lin, Y.H. Liou, Y. Zhang, C.L. Chen, C.-L. Dong, S.-Y. Chen, G.D. Stucky, Appl. Catal. B 127, 175–181 (2012)
J. Łuczak, M. Paszkiewicz, A. Krukowska, A. Malankowska, A. Zaleska-Medynska, Adv. Colloid Interface Sci. 230, 13–28 (2016)
J.R. Sivalingam, F.K. Chong, C.D. Wilfred, Bull. Chem. React. Eng. Catal. 13, 170–178 (2018)
A.R. Khataee, M. Zarei, S.K. Asl, J. Electroanal. Chem. 648, 2 (2010)
A. Ehsani, E. Kowsari, M. Dashti Najafi, R. Safari, H. Mohammad Shiri, J. Colloid Interface Sci. 503, 10–16 (2017)
A. Mobeen Amanulla, S.K. Jasmine Shahina, R. Sundaram, C. Maria Magdalane, K. Kaviyarasu, D. Letsholathebe, S.B. Mohamed, J. Kennedy, M. Maaza, J. Photochem. Photobiol. B 183, 233–241 (2018)
Y. Subba Reddy, C. Maria Magdalane, K. Kaviyarasu, G.T. Mola, J. Kennedy, M. Maaza, J. Phys. Chem. Solids 123, 43–51 (2018)
A. León, P. Reuquen, C. Garín, R. Segura, P. Vargas, P. Zapata, P. Orihuela, Appl. Sci. 7, 49 (2017)
K. Kasinathan, J. Kennedy, M. Elayaperumal, M. Henini, M. Malik, Sci. Rep. 6, 38064 (2016)
D.C.L. Vasconcelos, V.C.O. Costa, E.H.M. Nunes, A.N.C.S. Sabioni, M. Gasparon, W.L. Vasconcelos, Mater. Sci. Appl. 02, 1375–1382 (2011)
J. Zhu, F. Chen, J. Zhang, H. Chen, M. Anpo, J. Photochem. Photobiol. A 180, 196–204 (2006)
B. Pal, T. Hata, K. Goto, G. Nogami, J. Mol. Catal. A 169, 1 (2001)
R.H. Myers, D.C. Montgomery, C.M. Andreson-Cook, Response Surface Methodology: Process and Product Optimization Using Designed Experiments (Wiley, New York, 2016)
L. Yang, L.E. Yu, M.B. Ray, Water Res. 42, 13 (2008)
X. Zhang, F. Wu, X. Wu, P. Chen, N. Deng, J. Hazard. Mater. 157, 2 (2008)
C. Maria Magdalane, K. Kaviyarasu, J. Judith Vijaya, B. Siddhardha, B. Jeyaraj, J. Photochem. Photobiol. B 173, 23–34 (2017)
R. Suich, J. Qual. Technol. 12, 4 (1980)
Acknowledgments
This research was conducted at the Environmental Health Engineering Research Center and sponsored by the Vice-Chancellor for Research and Technology of the Kerman University of Medical Sciences. A note of appreciation is expressed here to the Vice-Chancellor and to all the University’s staff who provided assistance in the process of conducting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheikh Asadi, A.M., Malakootian, M. Preparation and characterization of Fe/TiO2 in the presence of ionic liquid to optimize the photocatalytic degradation of acetaminophen using the response surface methodology. J Mater Sci: Mater Electron 30, 14878–14889 (2019). https://doi.org/10.1007/s10854-019-01859-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01859-z