Abstract
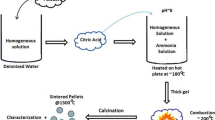
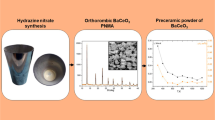
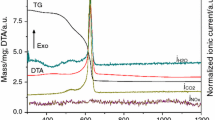
The precursor powder of 612 aluminates were synthesized by using liquid-phase co-precipitation method. The microstructure and formation mechanisms of the precursor powder were investigated through X-ray diffraction, thermal gravimetric, differential scanning calorimetry, and scanning electron microscope. The effects of the stacking states of the precursor powder, calcination temperature, and atmosphere on the phase compositions of 612 aluminates were also systematically studied. Results showed that the prepared precursor powder was a mixture of BaCO3, BaCa(CO3)2, and amorphous AlOOH, which with particle size ranging from 20 to 30 nm. The stacking states of the precursor powder considerably influenced the phase composition of aluminates. The pressed precursor tablet can ensure the final phase composition of aluminates was Ba3CaAl2O7. When the precursor powder was calcined in CO2 atmosphere at 1400 °C for 2 h, the phase composition included Ba5CaAl4O12, BaAl2O4, and BaCO3. When the calcination temperature was increased, the main crystalline phase of aluminates changed from Ba5CaAl4O12 to Ba3CaAl2O7 in flowing N2, Ar, and static air. The barium–tungsten cathode prepared by aluminates of Ba3CaAl2O7 phase showed better emissivity than that of Ba5CaAl4O12 phase. The current density of pulse emission at 1050 °C can reach 35.31 A/cm2.










Similar content being viewed by others
References
V.G. Vorozheikin, V.I. Kozlov, in IEEE International Vacuum Electron Sources Conference (2004), pp. 307–308
X. Wang, Y. Zhang, Y. Ding, X. Du, S. Qi, Q. Zhao, Y. Li, Q. Zhang, M. Meng, X. Hu, Zhenkong Kexue Yu Jishu Xuebao/Journal Vac. Sci. Technol. 35, 468 (2015)
J.X. Bao, B.F. Wan, P.J. Wang, Vacuum 81, 1029 (2007)
. C. Higashi, N.B. De Lima, J.R. Matos, C. Giovedi, in IEEE SBMO/IEEE MTT-S International Conference on Microwave and Optoelectronics (2005), pp. 222–225
L. Schoenbeck, Georg. Inst. Technol. (2005)
M. Shiran, M.J. Hadianfard, M.M. Shiezadeh, Int. J. Chem. Eng. Appl. 4, 88 (2013)
Q. Wang, W. Liu, L. Dong, X. Zhu, X. Liu, J.S. Wang, in IEEE Vacuum Electronics Conference (2015), pp. 1–2
C. Higashi, N.B. De Lima, J.R. Matos, C. Giovedi, C.C. Motta, in SBMO/IEEE MTT-S International Conference on Microwave and Optoelectronics, IEEE (2005), pp. 345–348
F.F. Sene, V.O. Santos, C.C. Motta, in IEEE Vacuum Electron Sources Conference (2012), pp. 165–166
K. Dudley, Vacuum 11, 84 (1961)
J.M. Roquais, F. Poret, R. Le Doze, J.L. Ricaud, A. Monterrin, A. Steinbrunn, Appl. Surf. Sci. 215, 5 (2003)
I.P. Melnikova, V.G. Vorozheikin, D.A. Usanov, Appl. Surf. Sci. 215, 59 (2003)
L.E. Branovich, D.W. Eckart, US 5298830 A. (1994)
I. Brodie, R.O. Jenkins, Br. J. Appl. Phys. 8, 27 (1957)
F.F. Sene, V.A. Mancini, V.O. Santos, C.C. Motta, in IEEE Vacuum Electronics Conference (2013), pp. 1–2
F.F. Sene, A.G.L. Silva, C.C. Motta, in IEEE Vacuum Electron Sources Conference (2012), pp. 183–184
R.A. Lipeles, H.K.A. Kan, Appl. Surf. Sci. 16, 189 (1983)
E.S. Rittner, W.C. Rutledge, R.H. Ahlert, J. Appl. Phys. 28, 1468 (1957)
K.F. Wang, L. Wei, J. Wang, Y. Cui, W. Xi, Rare Met. Mater. Eng. 42, 2326 (2013)
K.F. Wang, L. Wei, J.S. Wang, J. Inorg. Mater. 28, 1354 (2013)
H. Tian, Y.W. Liu, Y. Han, H.X. Hong, J.X. Yang, Z.Y. Xu, M.F. Meng, H.L. Zhang, J. Vac. Sci. Technol. 29, 64 (2009)
X. Wang, X. Liao, J. Luo, Q. Zhao, Chin. J. Vac. Sci. Technol. 24, 67 (2004)
M.G. Ma, Y.J. Zhu, J.F. Zhu, Z.L. Xu, Mater. Lett. 61, 5133 (2007)
C.A. Weiss, K.T. Cancel, R.D. Moser, P.G. Allison, E.R. Gore, M.Q. Chandler, P.G. Malone, J. Nanotechnol. Smart Mater. 105, 1 (2014)
H.S. Lee, H.H. Tai, K. Kim, Mater. Chem. Phys. 93, 376 (2005)
H. Liu, Z.H. Shi, Y.Q. Chen, B. Zhao, M.C. Gong, Chin. J. Inorg. Chem. 20, 688 (2004)
H. Kumazawa, K. Oki, H.M. Cho, E. Sada, Chem. Eng. Commun. 115, 25 (2007)
K.J. Mackenzie, J. Temuujin, M.E. Smith, P. Angerer, Y. Kameshima, Thermochim. Acta 359, 87 (2000)
S.M. Antao, I. Hassan, Phys. Chem. Miner. 34, 573 (2007)
I. Arvanitidis, D. Siche, S. Seetharaman, Metall. Mater. Trans. B 27, 409 (1996)
Chemical Industry Press Division, Chemical and Chemical Dictionary, 1st edn. (Chemical Industry Press, Beijing, 2003), pp. 2238–2239
I. Galan, F.P. Glasser, C. Andrade, J. Therm. Anal. Calorim. 111, 1197 (2013)
E.Q. Zhang, X.Q. Liu, J. Electron. Inf. Technol. 6, 89 (1984)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, J., Li, J., Feng, Y. et al. Factors affecting the phase composition of 612 aluminates for Ba–W cathode. J Mater Sci: Mater Electron 29, 16330–16337 (2018). https://doi.org/10.1007/s10854-018-9723-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9723-7