Abstract
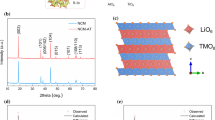
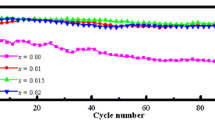
The cycle stability of Li(Ni0.8Co0.1Mn0.1)O2 is enhanced obviously by titanium doping via a facile solid-state method. The property of crystal structure is evaluated by XRD, which illustrates the samples possessed a layered α-NaFeO2 structure with R-3m space group. According to the charge/discharge studies, the capacity retention of pristine sample is around 51% after 125 cycles at 5 C, and the sample with Ti dopant displays a good cyclic stability, after 125 cycles, the capacity retention increases to 75% under 5 C, suggesting it could be possibly applied in fast charge Lithium-ion battery area. The superb electrochemical performance might be attributed to the Ti4+ occupy the layer structure to broaden the Lithium-ion channel, which is benefit to lithium intercalation and deintercalation during cycling.








Similar content being viewed by others
References
A.S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon, W. van Schalkwijk, Nat. Mater. 4:366 (2005). https://doi.org/10.1038/nmat1368
B. Dunn, H. Kamath, J.-M. Tarascon, Science 334, 928 (2011)
N.-S. Choi, Z. Chen, S.A. Freunberger et al., Angew. Chem. Int. Ed. 51, 9994 (2012). https://doi.org/10.1002/anie.201201429
S.-M. Bak, K.-W. Nam, W. Chang et al., Chem. Mater. 25, 337 (2013). https://doi.org/10.1021/cm303096e
S. Hwang, S.M. Kim, S.-M. Bak et al., ACS Appl. Mater. Interfaces 6, 15140 (2014). https://doi.org/10.1021/am503278f
Y.-K. Sun, S.-T. Myung, B.-C. Park, K. Amine, Chem. Mater. 18, 5159 (2006). https://doi.org/10.1021/cm061746k
X. Zheng, X. Li, B. Zhang et al., Ceram. Int. 42, 644 (2016). https://doi.org/10.1016/j.ceramint.2015.08.159
S.S. Jan, S. Nurgul, X. Shi, H. Xia, H. Pang, Electrochim. Acta 149, 86 (2014). https://doi.org/10.1016/j.electacta.2014.10.093
C. Hua, K. Du, C. Tan, Z. Peng, Y. Cao, G. Hu, J. Alloy. Compd. 614, 264 (2014). https://doi.org/10.1016/j.jallcom.2014.06.049
C. Zhang, J. Qi, H. Zhao et al., Mater. Lett. 201, 1 (2017). https://doi.org/10.1016/j.matlet.2017.04.121
Z. Huang, Z. Wang, Q. Jing, H. Guo, X. Li, Z. Yang, Electrochim. Acta 192, 120 (2016). https://doi.org/10.1016/j.electacta.2016.01.139
Y. Huang, F.-M. Jin, F.-J. Chen, L. Chen, J. Power Sources 256, 1 (2014). https://doi.org/10.1016/j.jpowsour.2014.01.003
K. Liu, G.-L. Yang, Y. Dong, T. Shi, L. Chen, J. Power Sources 281, 370 (2015). https://doi.org/10.1016/j.jpowsour.2014.12.131
S.U. Woo, C.S. Yoon, K. Amine, I. Belharouak, Y.K. Sun, J. Electrochem. Soc. 154, A1005 (2007)
S.W. Song, G.V. Zhuang, P.N. Ross, J. Electrochem. Soc. 151, A1162 (2004)
B.J. Neudecker, R.A. Zuhr, B.S. Kwak, J.B. Bates, J.D. Robertson, J. Electrochem. Soc. 145, 4148 (1998)
D.P. Abraham, R.D. Twesten, M. Balasubramanian, I. Petrov, J. McBreen, K. Amine, Electrochem. Commun. 4, 620 (2002). https://doi.org/10.1016/S1388-2481(02)00388-0
P.R. Ilango, T. Subburaj, K. Prasanna, Y.N. Jo, C.W. Lee, Mater. Chem. Phys. 158, 45 (2015). https://doi.org/10.1016/j.matchemphys.2015.03.033
K. Araki, N. Taguchi, H. Sakaebe, K. Tatsumi, Z. Ogumi, J. Power Sources 269, 236 (2014). https://doi.org/10.1016/j.jpowsour.2014.06.101
Y. Huang, Y. Huang, X. Hu, Electrochim. Acta 231, 294 (2017). https://doi.org/10.1016/j.electacta.2017.02.067
H. Meng, P. Zhou, Z. Zhang, Z. Tao, J. Chen, Ceram. Int. 43, 3885 (2017). https://doi.org/10.1016/j.ceramint.2016.12.054
Z. Qiu, Y. Zhang, P. Dong, S. Xia, Y. Yao, Solid State Ion. 307, 73 (2017). https://doi.org/10.1016/j.ssi.2017.04.011
X. Xiong, Z. Wang, X. Yin, H. Guo, X. Li, Mater. Lett. 110, 4 (2013). https://doi.org/10.1016/j.matlet.2013.07.098
L. Li, Y. Cao, H. Zheng, C. Feng, J. Mater. Sci.:Mater. Electron. 28, 1925 (2017). https://doi.org/10.1007/s10854-016-5745-1
X. Lu, X. Li, Z. Wang, H. Guo, G. Yan, X. Yin, Appl. Surf. Sci. 297, 182 (2014). https://doi.org/10.1016/j.apsusc.2014.01.121
G.-W. Yoo, B.-C. Jang, J.-T. Son, (2015) Ceram. Int. 41:1913. https://doi.org/10.1016/j.ceramint.2014.09.077
J.J. Saavedra-Arias, N.K. Karan, D.K. Pradhan et al., J. Power Sources 183, 761 (2008). https://doi.org/10.1016/j.jpowsour.2008.05.068
K.M. Shaju, G.V. Subba Rao, B.V.R. Chowdari, Electrochim. Acta 48, 145 (2002). https://doi.org/10.1016/S0013-4686(02)00593-5
H. Liu, J. Li, Z. Zhang, Z. Gong, Y. Yang, Electrochim. Acta 49, 1151 (2004). https://doi.org/10.1016/j.electacta.2003.11.001
L. Croguennec, E. Suard, P. Willmann, C. Delmas, Chem. Mater. 14, 2149 (2002). https://doi.org/10.1021/cm011265v
Y. Yao, H. Liu, G. Li, H. Peng, K. Chen, Electrochim. Acta 113, 340 (2013). https://doi.org/10.1016/j.electacta.2013.09.071
F. Wu, M. Wang, Y. Su, S. Chen, B. Xu, J. Power Sources 191, 628 (2009). https://doi.org/10.1016/j.jpowsour.2009.02.063
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 1474057, 51774076 and 51704063) and the Fundamental Research Funds for the Central Universities (N162502003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Li, Y., Guo, Y. et al. A facile method to enhance electrochemical performance of high-nickel cathode material Li(Ni0.8Co0.1Mn0.1)O2 via Ti doping. J Mater Sci: Mater Electron 29, 10702–10708 (2018). https://doi.org/10.1007/s10854-018-9093-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9093-1