Abstract
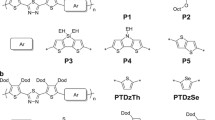
A series of novel benzothiadiazole based triphenylamine polymers containing different electron-rich side chains (dithienosilole, dithienopyrrole, dithieno-cyclopentadiene derivatives) have been prepared and their photoelectric properties explored. These polymers have been characterized using UV–Vis absorption and fluorescence spectroscopy and their photo-electrochemical properties characterized using cyclic voltammetry. These new polymers exhibit high thermal decomposition temperatures and broad absorption spectra that enable them to absorb light across the entire visible region. The UV absorption response range electron of these polymers could be tuned by modifying their electron donating units, with the best polymer found to exhibit a long fluorescent lifetime. An P3:PC71BM device containing dithienocyclopentadiene electron donating side-chains was shown to give 3.6% photoelectric conversion efficiency for a high short-circuit current of 14.25 mA/cm2. The fluorescence intensity of this copolymer decayed rapidly, implying that effective charge transfer processes were occurring at its interface, which were associated with a relatively high short circuit current. This work clearly demonstrates that use of an appropriate electron donor unit can effectively broaden the polymers spectroscopic response range, thus increasing the rate of carrier transport at the interface, which leads to improved photoelectric conversion efficiency.









Similar content being viewed by others
References
L. Ye, Y. Xiong, Q. Zhang, S. Li, C. Wang, Z. Jiang, J. Hou, W. You, H. Ade, Surpassing 10% efficiency benchmark for nonfullerene organic solar cells by scalable coating in air from single nonhalogenated solvent. Adv. Mater. 30(8), 1705485 (2018)
F. Zhao, C. Wang, X. Zhan, Morphology control in organic solar cells. Adv. Energy Mater. 8, 1703147 (2018)
G. Han, Y. Yi, Z. Shuai, From molecular packing structures to electronic processes: theoretical simulations for organic solar cells. Adv. Energy Mater. 8, 1702743 (2018)
L. Ye, B.A. Collins, X. Jiao, J. Zhao, H. Yan, H. Ade, Miscibility–function relations in organic solar cells: significance of optimal miscibility in relation to percolation. Adv. Energy Mater. 8, 1703058 (2018)
K. Zhang, B. Fan, R. Xia, X. Liu, Z. Hu, H. Gu, S. Liu, H.L. Yip, L. Ying, F. Huang, Highly efficient tandem organic solar cell enabled by environmentally friendly solvent processed polymeric interconnecting layer. Adv. Energy Mater. 8, 1703180 (2018)
S. Gunes, H. Neugebauer, N.S. Sariciftci, Conjugated polymer-based organic solar cells. Chem. Rev. 38, 1324–1338 (2007)
D. Xiao, Ternary organic solar cells offer 14% power conversion efficiency. Sci. Bull. 62, 1562–1564 (2017)
Y. Cui, H.F. Yao, C.Y. Yang, S.Q. Zhang, J.H. Hou, Organic solar cells with an efficiency approaching 15%. Acta Polym. Sin. 2, 223–230 (2018)
Z. Ding, Z. Miao, Z. Xie, J. Liu, Functionalized graphene quantum dots as a novel cathode interlayer of polymer solar cells. J. Mater. Chem. A 4(7), 2413–2418 (2016)
J.A. Amonoo, A. Li, G.E. Purdum, M.E. Sykes, B. Huang, An all-conjugated gradient copolymer approach for morphological control of polymer solar cells. J. Mater. Chem. A 3(40), 20174–20184 (2015)
D. Liu, B. Yang, B. Jang, B. Xu, S. Zhang, C. He, Y.W. Han, J. Hou, Molecular design of a wide-band-gap conjugated polymer for efficient fullerene-free polymer solar cells. Energy Environ. Sci. 10(2), 546–551 (2017)
D. Yang, Z. Guan, Y. Lin, H. Yan, Q. Wei, Z. Lu, J. Yu, Novel high-performance photovoltaic D–A conjugated polymers bearing 1,2-squaraine moieties as electron-deficient units. Sol. Energy Mater. Sol. Cells 105(105), 220–228 (2012)
H. Shi, L. Du, W. Xiong, M. Dai, W.K. Chan, D.L. Phillips, Study of electronic interactions and photo-induced electron transfer dynamics in a metalloconjugated polymer–single-walled carbon nanotube hybrid by ultrafast transient absorption spectroscopy. J. Mater. Chem. A 5, 18527–18534 (2017)
Y. Qin, S. Liu, H. Gu, W. Dai, X. Luo, Highly flattened donor-acceptor polymers based on fluoride-substituent acceptors for efficient heterojunction solar cells. Sol. Energy 166, 450–457 (2018)
M. Zhang, X. Guo, Y. Li, Synthesis and characterization of a copolymer based on thiazolothiazole and dithienosilole for polymer solar cells. Adv. Energy Mater. 1(4), 557–560 (2011)
Z. Jing, Z. Yao, Y. Cai, Y. Lin, M. Xu, R. Li, Z. Min, X. Dong, W. Peng, Conjugated linker correlated energetics and kinetics in dithienopyrrole dye-sensitized solar cells. Energy Environ. Sci. 6(5), 1604–1614 (2013)
I. Etxebarria, J. Ajuria, R. Pacios, Polymer:fullerene solar cells: materials, processing issues, and cell layouts to reach power conversion efficiency over 10%, a review. J. Photon. Energy 5(1), 057214 (2015)
J.T. Bloking, X. Han, A.T. Higgs, J.P. Kastrop, L. Pandey, J.E. Norton, C. Risko, C.E. Chen, J.L. Brédas, M.D. Mcgehee, Solution-processed organic solar cells with power conversion efficiencies of 2.5% using benzothiadiazole/imide-based acceptors. Chem. Mater. 23(24), 5484–5490 (2016)
A.K. Jin, E. Lim, K.K. Lee, S. Lee, S.H. Kim, A benzothiadiazole-based oligothiophene for vacuum-deposited organic photovoltaic cells. Sol. Energy Mater. Sol. Cells 94(12), 2057–2063 (2010)
R.Y. Lin, C.P. Lee, Y.C. Chen, J.D. Peng, T.C. Chu, H.H. Chou, H.M. Yang, J.T. Lin, K.C. Ho, Benzothiadiazole-containing donor-acceptor-acceptor type organic sensitizers for solar cells with ZnO photoanodes. Chem. Commun. 48(99), 12071 (2012)
C. Rodriguez-Seco, S. Biswas, G.D. Sharma, A. Vidal-Ferran, E. Palomares, Benzothiadiazole substituted semiconductor molecules for organic solar cells: the effect of the solvent annealing over the thin film hole mobility values. J. Phys. Chem. C (2018). https://doi.org/10.1021/acs.jpcc.8b0084
P. Agarwala, D. Kabra, A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): evolution and molecular engineering. J. Mater. Chem. A 5(4), 1348–1373 (2016)
S.P. Singh, M.S. Roy, K.R.J. Thomas, S. Balaiah, K. Bhanuprakash, G.D. Sharma, New triphenylamine (TPA) based organic dyes with different number of anchoring groups for dye-sensitized solar cells. J. Phys. Chem. C 116(9), 5941–5950 (2012)
K. Sulaiman, M.S. Fakir, Electrical conduction and photovoltaic effects of TPA-derivative solar cells. Thin Solid Films 519(15), 5219–5222 (2011)
H. Shang, K. Jiang, X. Zhan, An oligothiophene dye with triphenylamine as side chains for efficient dye-sensitized solar cells. Org. Electron. 13(11), 2395–2400 (2012)
J. Du, E. Xu, H. Zhong, F. Yu, C. Liu, H. Wu, D. Zeng, S. Ren, J. Sun, Y. Liu, Alkyl side chain driven tunable red–yellow–green emission: Investigation on the new π-conjugated polymers comprising of 2,7-carbazole unit and 2,1,3-benzo-thiadiazole units with different side chains. J. Polym. Sci. A 46(4), 1376–1387 (2008)
Y. Lin, Y. Chen, T. Ye, D. Ma, Y. Li, Carbazole-modified blue light-emitting copolymers with the backbones integrated by diphenyloxadiazole, fluorene, and triphenylamine. Eur. Polym. J. 48(2), 416–424 (2012)
Y. Zhi, B. Zhao, R. Cao, Y. Xu, J. Wang, D. Dang, C. Gao, L. Meng, Triphenylamine cored electron-donors for solution-processed organic solar cells: from tri-armed molecules to tetra-armed molecules. Dyes Pigm. 153, 291–299 (2018)
C. Goh, R.J. Kline, M.D. Mcgehee, E.N. Kadnikova, J.M.J. Fréchet, Molecular-weight-dependent mobilities in regioregular poly (3-hexyl-thiophene) diodes. Appl. Phys. Lett. 86, 122110 (2005)
Y. Liu, X. Wan, F. Wang, J. Zhou, G. Long, J. Tian, Y. Chen, High-performance solar cells using a solution-processed small molecule containing benzodithiophene unit. Adv. Mater. 23, 5387 (2011)
G. Zhao, Y. He, Z. Xu, J. Hou, M. Zhang, J. Min, H.Y. Chen, M. Ye, Z. Hong, Y. Yang, Effect of carbon chain length in the substituent of PCBM-like molecules on their photovoltaic properties. Adv. Funct. Mater. 20, 1480 (2010)
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (51663018), Outstanding Youth Funds of Jiangxi Province (20171BCB23056), and Natural Science Foundation of Jiangxi Province (20161BBG70043).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Deng, W., Qin, Y., Lin, S. et al. Side chain triphenylamine-based conjugated polymers for the preparation of efficient heterojunction solar cells. J Mater Sci: Mater Electron 30, 2235–2245 (2019). https://doi.org/10.1007/s10854-018-0495-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0495-x