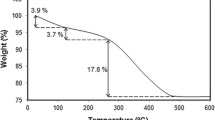
Abstract
A simple one step co-precipitation route was employed to synthesize the size controlled quantum dots (size ~ 3 nm) by maintaining the pH value at 6, without addition of capping agent. The effect of different annealing temperatures on the structural and optical properties of as synthesized sample have been explained. XRD analysis of sample showed the formation of pure rutile SnO2 quantum dots with tetragonal rutile structure for all the prepared samples. The SnO2 sample prepared by co-precipitation route was annealed at different temperatures and photo catalytic activities of annealed samples at various temperatures were investigated under direct solar irradiation. The obtained prepared sample with smallest size (3 nm) annealed at 300 °C displayed excellent degradation of Eosin Y (EY) dye. This is attributed to small grain size, tuned band gap, high crystallinity and high interfacial surface area. Quantum confinement effect enhanced the catalyst performance by creating oxygen vacancies and enabling the efficient charge transportation through discrete energy levels. Synthesized samples of SnO2 was subjected to the photocatalysis experiment to determine their photodegradation efficiency and was found to be lower for annealed samples than that shown by SnO2 quantum dots. QDs showed 73% degradation efficiency for EY dye when illuminated under sunlight for an hour.
















Similar content being viewed by others
References
Y. Zhao, Z. Zhang, H. Dang, Preparation of tin nanoparticles by solution dispersion. Mater. Sci. Eng. A 359, 405–407 (2003). https://doi.org/10.1016/S0921-5093(03)00395-2
V. Inderan, S.Y. Lim, T.S. Ong, S. Bastien, N. Braidy, H.L. Lee, Synthesis and characterisations of SnO2 nanorods via low temperature hydrothermal method. Superlattices Microstruct. 88, 396–402 (2015). https://doi.org/10.1016/j.spmi.2015.09.031
R. Adnan, N.A. Razana, I.A. Rahman, M.A. Farrukh, Synthesis and characterization of high surface area tin oxide nanoparticles via the sol–gel method hydrogenation styrene. J. Chin. Chem. Soc. 57 222–229 (2010)
T. Krishnakumar, N. Pinna, K.P. Kumari, K. Perumal, R. Jayaprakash, Microwave-assisted synthesis and characterization of tin oxide nanoparticles. Mater. Lett. 62, 3437–3440 (2008). https://doi.org/10.1016/j.matlet.2008.02.062
S.-C. Lee, J.-H. Lee, T.-S. Oh, Y.-H. Kim, Fabrication of tin oxide film by sol–gel method for photovoltaic solar cell system. Sol. Energy Mater. Sol. Cells 75, 481–487 (2003). https://doi.org/10.1016/S0927-0248(02)00201-5
S. Schiller, U. Heisig, K. Steinfelder, J. Strümpfel, R. Voigt, R. Fendler et al., On the investigation of d.c. plasmatron discharges by optical emission spectrometry. Thin Solid Films 96, 235–240 (1982). https://doi.org/10.1016/0040-6090(82)90247-4
R.S. Niranjan, Y.K. Hwang, D.K. Kim, S.H. Jhung, J.S. Chang, I.S. Mulla, Nanostructured tin oxide: synthesis and gas-sensing properties. Mater. Chem. Phys. 92, 384–388 (2005). https://doi.org/10.1016/j.matchemphys.2005.01.050
M. Aziz, S. Saber Abbas, W.R. Wan, Baharom, Size-controlled synthesis of SnO2 nanoparticles by sol–gel method. Mater. Lett. 91, 31–34 (2013). https://doi.org/10.1016/j.matlet.2012.09.079
T. Stergiopoulos, I.M. Arabatzis, H. Cachet, P. Falaras, Photoelectrochemistry at SnO2 particulate fractal electrodes sensitized by a ruthenium complex: solid-state solar cell assembling by incorporating a composite polymer electrolyte. J. Photochem. Photobiol. A 155, 163–170 (2003). https://doi.org/10.1016/s1010-6030(02)00394-5
Y. Liu, W. Yang, Z. Dai, H. Chen, X. Yang, D. Hou, Improved molten salt synthesis (MSS) for SnO2 nanorods and nanotwins. Mater. Chem. Phys. 112, 381–386 (2008). https://doi.org/10.1016/j.matchemphys.2008.05.064
J. Kong, H. Deng, P. Yang, J. Chu, Synthesis and properties of pure and antimony-doped tin dioxide thin films fabricated by sol–gel technique on silicon wafer. Mater. Chem. Phys. 114, 854–859 (2009). https://doi.org/10.1016/j.matchemphys.2008.10.049
J. Zhang, L. Gao, Synthesis and characterization of nanocrystalline tin oxide by sol-gel method. J. Solid State Chem. 177, 1425–1430 (2004). https://doi.org/10.1016/j.jssc.2003.11.024
C. Fu, J. Wang, M. Yang, X. Su, J. Xu, B. Jiang, Effect of la doping on microstructure of SnO2 nanopowders prepared by co-precipitation method. J. Non Cryst. Solids 357, 1172–1176 (2011). https://doi.org/10.1016/j.jnoncrysol.2010.10.019
M. Krishna, S. Komarneni, Conventional-vs microwave-hydrothermal synthesis of tin oxide, SnO2 nanoparticles. Ceram. Int. 35, 3375–3379 (2009). https://doi.org/10.1016/j.ceramint.2009.06.010
N.S. Baik, G. Sakai, N. Miura, N. Yamazoe, Preparation of stabilized nanosized tin oxide particles by hydrothermal treatment. J. Am. Ceram. Soc. 83, 2983–2987 (2000). https://doi.org/10.1111/j.1151-2916.2000.tb01670.x
S. Supothina, R. Rattanakam, S. Vichaphund, P. Thavorniti, Effect of synthesis condition on morphology and yield of hydrothermally grown SnO2 nanorod clusters. J. Eur. Ceram. Soc. 31, 2453–2458 (2011). https://doi.org/10.1016/j.jeurceramsoc.2011.02.018
H. Yang, Y. Hu, A. Tang, S. Jin, G. Qiu, Synthesis of tin oxide nanoparticles by mechanochemical reaction. J. Alloy. Compd. 363, 271–274 (2004). https://doi.org/10.1016/S0925-8388(03)00473-0
J.S. Lee, S.C. Choi, Solvent effect on synthesis of indium tin oxide nano-powders by a solvothermal process. J. Eur. Ceram. Soc. 25, 3307–3314 (2005). https://doi.org/10.1016/j.jeurceramsoc.2004.08.022
S. Mosadegh Sedghi, Y. Mortazavi, A. Khodadadi, Low temperature CO and CH4 dual selective gas sensor using SnO2 quantum dots prepared by sonochemical method. Sens. Actuators B 145, 7–12 (2010). https://doi.org/10.1016/j.snb.2009.11.002
N. Sharma, R. Jha, S. Baghel, D. Sharma, Study on photocatalyst zinc oxide annealed at different temperatures for photodegradation of Eosin Y dye. J. Alloy. Compd. 695, 270–279 (2017). https://doi.org/10.1016/j.jallcom.2016.10.194
N.D. Hoa, N. Van Quy, H. Song, Y. Kang, Y. Cho, D. Kim, Tin oxide nanotube structures synthesized on a template of single-walled carbon nanotubes. J. Cryst. Growth 311, 657–661 (2009). https://doi.org/10.1016/j.jcrysgro.2008.09.076
E.S.M.A. Duraia, Z.A. Mansurov, S.Z. Tokmoldin, V.V. Klimenov, I.S. Nevmerzhitsky, A.M. Dochshanov, Synthesis and characterization of tin oxide nanoribbons and nanowires, in: Proceedings of International Siberian Conference on Control and Communications, SIBCON-2009, pp. 211–215 (2009). https://doi.org/10.1109/SIBCON.2009.5044858
A. Kolmakov, Y. Zhang, G. Cheng, M. Moskovits, Detection of CO and O2 using tin oxide nanowire sensors. Adv. Mater. 15, 997–1000 (2003). https://doi.org/10.1002/adma.200304889
P. Chetri, P. Basyach, A. Choudhury, Structural, optical and photocatalytic properties of TiO2/SnO2 and SnO2/TiO2 core–shell nanocomposites: an experimental and DFT investigation. Chem. Phys. 434, 1–10 (2014). https://doi.org/10.1016/j.chemphys.2014.02.007
A. Bhattacharjee, M. Ahmaruzzaman, A novel and green process for the production of tin oxide quantum dots and its application as a photocatalyst for the degradation of dyes from aqueous phase. J. Colloid Interface Sci. 448, 130–139 (2015). https://doi.org/10.1016/j.jcis.2015.01.083
H.J. Wang, F.Q. Sun, Y. Zhang, L.S. Li, H.Y. Chen, Q.S. Wu et al., Photochemical growth of nanoporous SnO2 at the air–water interface and its high photocatalytic activity. J. Mater. Chem. 20, 5641–5645 (2010). https://doi.org/10.1039/b926930d
K. Vijayarangamuthu, S. Rath, Nanoparticle size, oxidation state, and sensing response of tin oxide nanopowders using Raman spectroscopy. J. Alloy. Compd. 610, 706–712 (2014). https://doi.org/10.1016/j.jallcom.2014.04.187
K. Vinodgopal, P.V. Kamat, Enhanced rates of photocatalytic degradation of an azo dye using SnO2/TiO2 coupled semiconductor thin films. Environ. Sci. Technol. 29, 841–845 (1995). https://doi.org/10.1021/es00003a037
A.K. Sinha, P.K. Manna, M. Pradhan, C. Mondal, S.M. Yusuf, T. Pal, Tin oxide with a p–n heterojunction ensures both UV and visible light photocatalytic activity. RSC Adv. 4, 208–211 (2014). https://doi.org/10.1039/C3RA42740D
L.E. Brus, Optical propagation and vibrational energy loss in gaseous waveguide lasers. IEEE J. Quantum Electron. 17, 293–295 (1981). https://doi.org/10.1109/JQE.1981.1071088
J. Moore, R. Louder, C. Thompson, Photocatalytic activity and stability of porous polycrystalline ZnO thin-films grown via a two-step thermal oxidation process. Coatings 4, 651–669 (2014). https://doi.org/10.3390/coatings4030651
H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri, J. Ye, Nano-photocatalytic materials: possibilities and challenges. Adv. Mater. 24, 229–251 (2012). https://doi.org/10.1002/adma.201102752
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, N., Jha, R. & Sharma, R. Growth of size-controllable tetragonal rutile stannic oxide nanostructures by co-precipitation route for eosin Y dye degradation under solar radiation. J Mater Sci: Mater Electron 29, 4801–4816 (2018). https://doi.org/10.1007/s10854-017-8436-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8436-7