Abstract
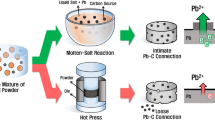
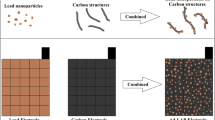
To enhance the power and energy densities of advanced lead–acid batteries (Ad-LAB), a novel core–shell structure of lead-activated carbon (Pb@AC) was prepared and used as a negative electrode active material. The AC could be formed as a shell around a core of Pb nanoparticles. The active core–shell structures were synthesized using a simple chemical process to overcome the limitations in the negative Pb electrode, specifically the large crystallization of lead sulfate (PbSO4) that leads to a short cycle life. The key role of the carbon material in the negative electrode is to enhance the electrochemical performances and decrease the formation of PbSO4. The X-ray diffraction study reveals that the formation of lead oxide was prevented by the AC during the synthetic process. The novel core–shell structure of Pb@AC was confirmed through transmission electron microscopy. In order to obtain high-performance Ad-LAB, a high surface area of the AC is necessary to provide a super capacitive effect in the negative electrode. The unit cell performance of the as-prepared active materials exhibits significant increased discharge capacity at 1C rate. The unit cell with the Pb@AC negative electrode has a capacity per unit volume of 0.0165 Ah/cc. Hence, the low cost of AC and the simple synthetic core–shell structure of Pb@AC make this material a promising negative electrode active material for Ad-LAB applications.








Similar content being viewed by others
References
S. Amjad, S. Neelakrishnan, R. Rudramoorthy, Review of design considerations and technological challenges for successful development and deployment of plug-in hybrid electric vehicles. Renew. Sustain. Energy Rev. 14, 1104 (2010)
X. Tian, Y. Gong, W. Yufeng, A. Agyeiwaa, T. Zuo, Management of used lead acid battery in china: Secondary lead industry progress, policies and problems. Resour. Conserv. Recycl. 93, 75 (2014)
C.C. Chan, The State of the Art of Electric, Hybrid and Fuel Cell Vehicles, Proceeding to IEEE, vol. 95 (2007), p 704
D. Pavlov, P. Nikolov, T. Rogachev, Influence of expander components on the processes at the negative plates of lead -acid cells on high-rate partial-state-of charching cycling. Part II. Effect of carbon additives on the processes of charge and discharge of negative plates. J. Power Sources 195, 4444 (2010)
A. Banerjee, R. Srinivasan, K.A. Shukla, Effects of carbon additives on the performance of negative electrode of lead-carbon battery. ECS Electrochem. Lett 3, A1 (2014)
L.T. Lam, R. Louey, Development of ultra-battery for hybrid -electric vehicle applications. J. Power Sources 158, 1140 (2006)
G. Wang, L. Zhang, J. Zhang, A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 41, 797 (2012)
M. Conte, A. Genovese, F. Ortenzi, F. Vellucci, Hybrid battery-supercapacitor storage for an electric forklift: a life-cycle cost assessment. J. Appl. Electrchem. 44, 523 (2014)
B.H. Patil, R.N. Bulakhe, C.D. Lokhande, Supercapacitive performance of chemically synthesized polypyrrole thin films: effect of monomer to oxidant ratio. J. Mater. Sci. 25, 2188 (2014).
F. Cheng, J. Liang, Z. Tao, J. Chen, Functional materials for rechargeable batteries. Adv. Mater. 23, 1695 (2011)
D. Sheng Su, R. Schogl, Nanosctructured carbon and carbon nanocomposite for electrochemical energy storage applications. ChemSusChem 3, 136 (2010)
P. Moseley, Consequences of including carbon in the negative plates of Valve-regulated lead-acid batteries exposed to high-rate partial-state of-charge operation. J. Power Sources 191, 134 (2009)
E. Ebner, A. Borger, M. Gelbke, E. Zena, M. Wieger, Temperature-dependent formation of vertical concentration gradients in lead-acid batteries under PSoC operation—part 1: acid stratification. Electrochim. Acta 90, 219 (2013)
S.W. Swogger, P. Everill, D.P. Dubey, N. Sugumaran, Discrete carbon nanotubes increase lead acid battery charge acceptance and performance. J. Power Sources 261, 55 (2014)
D. Pavlov, P. Nikolov, Capacitive carbon and electrochemical lead electrode systems at the negative plates of lead-acid batteries and elementary processes on cycling. J. Power Sources 242, 380 (2013)
P. Zolotavin, P.G. Sionnest, Meissner effect in colloidal Pb nanoparticles. ACS Nano 4, 5599 (2010)
T. Sadhasivam, K. Dhanabalan, S.-H. Roh, S.-C. Kim, D. Jeon, J.-E. Jin, J. Shim, H.-Y. Jung, Preparation and characterization of Pb nanoparticles on mesoporous carbon nanostructure for advanced lead-acid battery applications. J. Mater. Sci. (2017). doi:10.1007/s10854-016-6238-y
W. Zhang, H. Lin, H. Lu, D. Liu, J. Yin, Z. Lin, On the electrochemical origin of the enhanced charge acceptance of the lead-carbon electrode. J. Mater. Chem. A 3, 4399 (2015)
Acknowledgements
This research work was supported by basic science research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015061146), Korea Electric Power Corporation (KEPCO) (R15XA03-30) and Gwangju Green Environment Center (14-4-70-79).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhanabalan, K., Sadhasivam, T., Kim, S.C. et al. Novel core–shell structure of a lead-activated carbon (Pb@AC) for advanced lead–acid battery systems. J Mater Sci: Mater Electron 28, 10349–10356 (2017). https://doi.org/10.1007/s10854-017-6804-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6804-y