Abstract
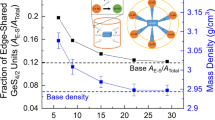
The aim of the present work is to investigate the effects of SiO2 content on the structure of CaO–B2O3–SiO2–Al2O3–ZnO glass using the MAS-NMR, the fourier transform infrared spectrometer and differential scanning calorimetry (DSC). The results showed that the majority of Al existed in fourfold coordination and Zn acted as glass modifier in glass structure. With the increasing of SiO2 content, the relative amount of BIII units decreased while the BIV units increased and the proportion of Si–O–Si bridge oxygen bond in [SiO4] tetrahedrons increased obviously. The DSC curves revealed that the glass transition temperature increased from 732 to 773 °C with the increasing of SiO2 content, indicating that SiO2 generating more bridge oxygen can increase the degree of polymerization of glass structure.




Similar content being viewed by others
References
G. Shao, X. Wu, Y. Kong, X. Shen, S. Cui, X. Guan, C. Jiao, J. Jiao, J. Alloy Compd. 663, 360 (2016)
M.Y. Hassaan, H.M. Osman, H.H. Hassan, A.S. El-Deeb, M.A. Helal, Ceram. Int. (2016). doi:10.1016/j.ceramint.2016.10.137
M. Klinger-Strobel, O. Makarewicz, M.W. Pletz, A. Stallmach, C. Lautenschlager, J. Mater. Sci. 27, 175 (2016)
M. Sitarz, J. Non-Cryst. Solids 357, 1603 (2011)
M. Ma, Z. Liu, F. Zhang, F. Liu, Y. Li, R. Bordia, J. Am. Ceram. Soc. 99, 2402 (2016)
Y. Lai, Y. Zeng, X. Tang, H. Zhang, J. Han, H. Su, RSC Adv. 6, 93722 (2016)
S. Khan, G. Kaur, K. Singh, Ceram. Int. 43, 722 (2017)
M. LaComb, D. Rice, J.F. Stebbins, J. Non-Cryst. Solids 447, 248 (2016)
J.Z. Liu, X.F. Wu, N.X. Xu, Q.L. Zhang, H. Yang, J. Mater. Sci. 26, 8899 (2015)
T.R. Rao, C.V. Reddy, C.R. Krishna, U.S.U. Thampy, R.R. Raju, P.S. Rao, R.V.S.S.N. Ravikumar, J. Non-Cryst. Solids 357, 3373 (2011)
R. Stefan, E. Culea, P. Pascuta, J. Non-Cryst. Solids 358, 839 (2012)
S. Cetinkaya Colak, I. Akyuz, F. Atay, J. Non-Cryst. Solids 432, 406 (2016)
J.S. Park, Y. Kim, H. Shin, J.H. Moon, W. Lim, J. Am. Ceram. Soc. 91, 3630 (2008)
K. Herzog, J. Peters, B. Thomas, C. Jäger, Ber. Bunsenges. Phys. Chem 100, 1655 (1996)
A. Gaddam, H.R. Fernandes, J.M.F. Ferreira, RSC Adv. 5, 41066 (2015)
B.G. Parkinson, D. Holland, M.E. Smith, A.P. Howes, C.R. Scales, J. Phys. 19, 415114 (2007)
S.H. Risbud, R.J. Kirkpatrick, A.P. Taglialavore, B. Montez, J. Am. Ceram. Soc. 70, C-10(1987)
S. Sen, Z. Xu, J. Stebbins, J. Non-Cryst. Solids 226, 29 (1998)
A. Saini, A. Khanna, V.K. Michaelis, S. Kroeker, F. González, D. Hernández, J. Non-Cryst. Solids 355, 2323 (2009)
K. Singh, I. Bala, V. Kumar, Ceram. Int. 35, 3401(2009)
A. Aronne, S. Esposito, P. Pernice, Phys. Chem. Glasses 40, 63 (1999)
X. Zhu, C. Mai, M. Li, J. Non-Cryst. Solids 388, 55 (2014)
M. Nakamura, Y. Mochizuki, K. Usami, Y. Itoh, T. Nozaki, Solid State Commun. 50, 1079 (1984)
N. Santha, T. Nideep, S. Rejisha, J. Mater. Sci. 23, 1435 (2012)
G.J. Mohini, N. Krishnamacharyulu, G. Sahaya Baskaran, P.V. Rao, N. Veeraiah, Appl. Surf. Sci 287, 46 (2013)
H. Shao, H.Q. Zhou, X.D. Shen, Adv. Mater. Res. 189, 4466 (2011)
J.H. Jean, C.R. Chang, C.D. Lei, J. Am. Ceram. Soc. 87, 1244 (2004)
C.R. Chang, J.H. Jean, J. Am. Ceram. Soc. 82, 1725 (1999)
Acknowledgements
The authors thank the National Center for Magnetic Resonance in Wuhan acquiring the MAS-NMR measurement. This work was supported by the fund of the State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals (Nos. SKL-SPM-201535, 201548), The 551 project of Kunming, the Basic Applied Research Foundation of Yunnan Province, China (Grant Nos. 2016FD125, 2016FB083) and Science &Technology Program of Yunnan Province (No. 2014DC019).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Han, J., Lai, Y., Xiang, Y. et al. Glass structure of the CaO–B2O3–SiO2–Al2O3–ZnO glasses system with different Si content. J Mater Sci: Mater Electron 28, 6131–6137 (2017). https://doi.org/10.1007/s10854-016-6291-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6291-6