Abstract
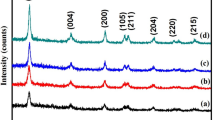
In this study, removal of methyl orange in aqueous media and visible light was investigated by using co-doped silver and copper TiO2 nanoparticles. Field emission scanning electron microscopy (FESEM), dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), UV–vis diffuse reflectance spectroscopy (DRS), Brunauer–Emmett–Teller (BET) and atomic force microscopy (AFM) were used for characterization of prepared nanoparticles. TiO2 nanoparticles were prepared by sol–gel method. Also silver and copper were doped in nanoparticles by impregnation method. Red shift occurred in co-doped Ag,Cu/TiO2 nanoparticles and exhibited highest photocatalytic activity compared to pure TiO2 nanoparticles. Optimum mole percentage of copper and silver for co-doped TiO2 nanoparticles were 0.03 and 1.3 respectively. Optimum co-doped TiO2 nanoparticles showed 50% degradation in the visible light within 40 min. The change in the diffraction angle (2θ), lattice parameter and the reduction in X-ray diffraction intensity, are the factors that proved silver and copper co-doped in TiO2 nanoparticles completely.








Similar content being viewed by others
References
W. Wunderlich, T. Oekermann, L. Miao, J. Ceram. Process Res. 5, 343–354 (2004)
S.S. Hong, M.S. Lee, C.S. Ju, G.D. Lee, S.S. Park, K.T. Lim, Catal. Today 93–95, 871–876 (2004)
M.A. Behnajady, H. Eskandarloo, N. Modirshahla, M. Shokri, Photobiol. 87, 1002–1008 (2011)
A. Fujishima, T.N. Rao, D.A. Tryk, J. Photochem. Photobiol. 1, 1–21 (2000)
A.T. Paxton, L. Thiên-Nga, Phys. Rev. B 57, 1579–1584 (1998)
S. Banerjee, J. Gopal, P. Muraleedharan, Curr. Sci. 90, 1378–1383 (2006)
Z. Wang, W. Ma, C. Chen, H. Ji, J. Zhao, J. Chem. Eng. 170, 353–362 (2011)
J. Saien, S. Khezrianjoo, J. Hazard. Mater. 157, 269–276 (2008)
M.A. Rauf, S.S. Ashraf, J. Chem. Eng. 151, 10–18 (2009)
S.N. Nalwa, Handbook of advanced electronic and photonic materials and devices, vol. 5, (Acadmic press, Cambridge, 2001)
H. Han, R. Ba, Ind. Eng. Chem Res. 48, 2891–2898 (2009)
O. Regan, M. Grätzel, Nature 353, 737–740 (1991)
M.R. Hoffmann, S.T. Martin, W. Choi, Chem. Rev. 95, 69–96 (1995)
A. Mills, A.J. Hunte, J Photochem. Photobiol. A. Chem. 108, 1–35 (1997)
Z. Ambrus, N. Balazs, T. Alapi, G. Wittmann, P. Sipos, A. Dombi, K. Mogyorosi, Appl. Catal. B Environ. 81, 27–37 (2008)
K. Song, J. Zhou, J. Bao, Y. Feng, J. Am. Ceram. Soc. 91, 1369–1371 (2008)
X.H. Wang, J.G. Li, H.K. Amiyama, Y. Moriyoshi, T. Ishigaki, J. Phys. Chem. B 110, 6804–6809 (2006)
C.C. Chan, C.C. Chang, W.C. Hsu, S.K. Wang, J. Lin, J. Chem. Eng. 152, 492–497 (2009)
O. Carp, C.L. Huisman, A. Reller, Prog. Sol. Sta. Chem. 32, 33–117 (2004)
G.K. Mor, O.K. Varghese, M. Paulose, Sol. Eng. Mater. Solar Cell 90, 2011–2075 (2006)
C. Burda, Y. Lou, X. Chen, Nano Lett. 3, 1049–1051 (2003)
Y. Choi, A. Termin, M.R. Hoffmann, J. Phys. Chem. 98, 13669–13679 (1994)
K. Song, J. Zhou, J. Bao, J. Am. Ceram. Soc. 91, 1369–1371 (2008)
A.A. Ashkarran, H. Hamidinezhad, H. Haddadi, Appl. Surf. Sci. 301, 338–345 (2014)
S. Naraginti, F.B. Stephen, A. Radhakrishnan, A. Sivakumar, Mol. Biomol. Spec. 135, 814–819 (2015)
Y. Yamada S. Shikano, T. Akita, S. Fukuzumi, Catal. Sci. Technol. 5, 979–988 (2015)
J. Talat-Mehrabad, M. Khosravi, N. Modirshahla, M.A. Behnajady, Des. Water. Treat. 57, 10451–10461 (2016)
S. Saha, J.M. Wang, A. Pal, Sep. Pur. Technol. 89, 147–159 (2012)
R.J. Tayade, R.G. Kulkarni, R.V. Jasra, Ind. Eng. Chem. Res. 45, 15 (2006)
B. Ohtani, Y. Ogawa, S. Nishimoto, J. Phys. Chem. B 101, 3746–3752 (1993)
N.N. Ilkhechi, B.K. Kaleji, J. Sol Gel. Sci. Technol. 69, 351–356 (2014)
I. Kostov Minerology, 3rd edn. Nauka, Izkustia, Sofia (1973)
Q. Wang, S. Xu, F. Shen, Appl. Surf. Sci. 257, 7671–7677 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalali, J., Mozammel, M. Degradation of water-soluble methyl orange in visible light with the use of silver and copper co-doped TiO2 nanoparticles. J Mater Sci: Mater Electron 28, 5336–5343 (2017). https://doi.org/10.1007/s10854-016-6192-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6192-8