Abstract
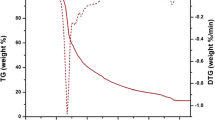
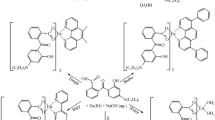
The luminescent binary and ternary europium(III) complexes were prepared by employing ethyl-(3-fluorobenzoyl) acetate (m-EFBA) as primary ligand and neocuproine (neo), bathophenanthroline (batho), 1,10-phenanthroline (phen) and 2,2-bipyridyl (bipy) as secondary ligands. The synthesized complexes Eu(m-EFBA)3·(H2O)2 (C1), Eu(m-EFBA)3·neo (C2), Eu(m-EFBA)3·batho (C3), Eu(m-EFBA)3·phen (C4), Eu(m-EFBA)3·bipy (C5) were characterized by the means of elemental analysis (C, H and N), nuclear magnetic resonance spectroscopy (1H-NMR), infrared spectroscopy (IR), thermogravimetric analysis (TG/DTG), UV–visible and photoluminescence (PL) spectroscopy. The photoluminescence spectra of complexes exhibit the characteristic emission band at 613 nm assigned to hypersensitive 5D0 → 7F2 transition, responsible for the red color emission of complexes. The higher photoluminescence intensity of ternary europium(III) complexes C2–C5 as compared to binary complex C1, suggest that ancillary ligands neo, batho, phen and bipy enhance the process of sensitization from ligand (m-EFBA) to europium(III) ion. The luminescence decay time and quantum efficiencies of the complexes were determined to estimate the efficiency of energy transfer from ligand to metal ion. In addition, the Judd–Ofelt intensity parameters (Ω2, Ω4) were calculated from the emission intensities of 5D0 → 7F2 and 5D0 → 7F4 transitions of europium(III) ion respectively. The intramolecular energy transfer mechanism of the complexes were also investigated and results indicate that the ligand (m-EFBA) and ancillary ligands effectively transfer the energy to that of Eu(III) ion.











Similar content being viewed by others
References
S.I. Weissman, J. Chem. Phys. 10, 214 (1942)
L.R. Melby, N.J. Rose, E. Abramson, J.C. Caris, J. Am. Chem. Soc. 86, 5117 (1964)
K. Binnemans, P. Lenaerts, K. Driesen, C. Görller-Walrand, J. Mater. Chem. 14, 191 (2004)
J.C. Bünzli, C. Piguet, Chem. Soc. Rev. 34, 1048 (2005)
G. Muller, Dalton Trans. 44, 9692 (2009)
S.V. Elisseva, J.C. Bünzli, Chem. Soc. Rev. 39, 189 (2010)
J.Q. Liu, Y.Y. Wang, S.R. Batten, H. Sakiyama, D.Y. Ma, Inorg. Chem. Commun. 19, 27 (2012)
X. Zhu, H.Y. Zheng, X.F. Wei, Z.Y. Lin, L.H. Guo, B. Qiu, G.N. Chen, Chem. Commun. 13, 1276 (2013)
L.X. Shi, C.D. Wu, Chem. Commun. 10, 2928 (2011)
Y.G. Lee, H.R. Moon, Y.E. Cheon, M.P. Suh, Angew. Chem. Int. Ed. 40, 7741 (2008)
T. Nitabaru, A. Nojiri, M. Kobayashi, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 131, 13860 (2009)
J.R. Morrow, Comments Inorg. Chem. 29, 169 (2008)
E.R. Farquhar, J.P. Richard, J.R. Morrow, Analog Inorg. Chem. 46, 7169 (2007)
V. Bekiari, P. Lianos, Adv. Mater. 10, 1455 (1998)
B. Gao, Z. Qiao, T. Chen, Mater. Chem. Phys. 143, 1119 (2014)
K. Binnemans, Chem. Rev. 109, 4283 (2009)
A.P. Souza, F.A.A. Paz, R.O. Freire, L.D. Carlos, O.L. Malta, S. Alves Jr., G.F. de Sà, J. Phys. Chem. B 111, 9228 (2007)
I.R. Lasker, T.M. Chen, Chem. Mater. 16, 111 (2004)
Z. Bao, A.J. Lovinger, J. Brown, J. Am. Chem. Soc. 120, 207 (1998)
C. Yang, L.M. Fu, Y. Wang, J.P. Zhang, W.T. Wong, X.C. Ai, Y.F. Qiao, B.S. Zou, L.L. Gui, Angew. Chem. Int. Ed. 43, 5010 (2004)
A. Dossing, Eur. J. Inorg. Chem. 2005, 1425 (2005)
M. Bala, S. Kumar, V.B. Taxak, P. Boora, S.P. Khatkar, J. Fluorine Chem. 178, 6 (2015)
E.J. Roh, J.M. Keller, Z. Olah, M.J. Ladarola, K.A. Jacobson, Bioorg. Med. Chem. 16, 9349 (2008)
D. Wang, C. Zheng, L. Fan, J. Zheng, X. Wei, Synth. Met. 162, 2063 (2012)
W. Zhang, C.H. Liu, R.R. Tang, C.Q. Tang, Bull. Korean Chem. Soc. 30, 2213 (2009)
M. Arvind, K. Sageed, Indian J. Chem. 25A, 589 (1986)
L. Fu, R.A.S. Ferreira, N.J.O. Silva, A.J. Fernandes, P. Ribeiro-Claro, S. Goncalves, V.D.Z. Bermudez, L.D. Carlos, J. Mater. Chem. 15, 3117 (2005)
Y. Zhang, H. Shi, Y. Ke, Y. Cao, J. Lumin. 124, 51 (2007)
D. Wang, C. Zheng, L. Fan, Y. Hu, J. Zheng, Spectrochim. Acta Mol. Biomol. Spectrosc. 117, 245 (2014)
M. Bala, S. Kumar, P. Boora, V.B. Taxak, A. Khatkar, S.P. Khatkar, J. Mater. Sci.: Mater. Electron. 25, 2850 (2014)
G. Shao, Y. Li, K. Feng, F. Gan, M. Gong, Sens. Actuators B 173, 692 (2012)
J. Kai, D.F. Parra, H.F. Brito, J. Mater. Chem. 18, 4549 (2008)
A.F. Kirby, D. Foster, F.S. Richardson, Chem. Phys. Lett. 95, 507 (1983)
F.S. Richardson, Chem. Rev. 82, 541 (1982)
R. Ilmi, K. Iftikar, Polyhedron 102, 16 (2015)
H. Wang, P. He, H. Yan, M. Gong, Sens. Actuators B 156, 6 (2011)
N. Sabbatini, M. Guardigli, J.M. Lehn, Coord. Chem. Rev. 123, 201 (1993)
H.F. Brito, O.L. Malta, L.R. Souza, J.F.S. Menezes, C.A.A. Carvalho, J. Non-Cryst, Solids 247, 129 (1999)
D.B.A. Raj, S. Biju, M.L.P. Reddy, Inorg. Chem. 47, 8091 (2008)
M.H.V. Werts, R.T.F. Jukes, J.W. Verhoeven, Phys. Chem. Chem. Phys. 4, 1542 (2002)
S. Stanimirov, I. Petkov, Spectrochim. Acta Mol. Biomol. Spectrosc. 72, 1127 (2009)
L.D. Carlos, Y. Messaddeq, H.F. Brito, R.A.S. Ferreira, V.D. Bermudez, S.J.L. Ribeiro, Adv. Mater. 12, 594 (2000)
R. Ferreira, P. Pires, B.D. Castro, R.A.S. Ferreira, L.D. Carlos, U. Pischel, N. J. Chem. 28, 1506 (2004)
G.F. de Sà, O.L. Malta, C. de Mello Donegà, A.M. Simas, R.L. Longo, P.A. Santa-Cruz, E.F. da Silva Jr., Coord. Chem. Rev. 196, 165 (2000)
M.C.F.C. Felinto, C.S. Tomiyama, H.F. Brito, E.E.S. Teotonio, O.L. Malta, J. Solid State Chem. 171, 189 (2003)
E.E.S. Teotonio, H.F. Brito, M.C.F.C. Felinto, C.A. Kodaira, O.L. Malta, J. Coord. Chem. 56, 913 (2003)
F. Cagnin, M.R. Davolos, E.E. Castellano, Polyhedron 67, 65 (2014)
P. Gawryszewska, J. Sokolnicki, J. Legendziewicz, Coord. Chem. Rev. 249, 2489 (2005)
M. Latva, H. Takalo, V.M. Mukkala, C. Matachescu, J.C.R. Ubis, J. Kankare, J. Lumin. 75, 149 (1997)
D.L. Dexter, J. Chem. Phys. 21, 836 (1953)
C.R.S. Dean, T.M. Shephred, J. Chem. Soc. Faraday Trans. 2(71), 146 (1975)
Acknowledgments
This work was financially supported in the form of senior research fellowship (SRF) from UGC, New Delhi, India (Award No: 2121210101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicated no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Devi, R., Dalal, M., Bala, M. et al. Synthesis, photoluminescence features with intramolecular energy transfer and Judd–Ofelt analysis of highly efficient europium(III) complexes. J Mater Sci: Mater Electron 27, 12506–12516 (2016). https://doi.org/10.1007/s10854-016-5760-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5760-2