Abstract
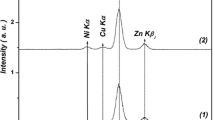
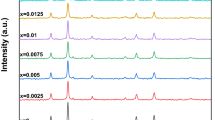
Impacts of hydrogen annealing on crystallographic characterization, electronic structure, and optical, photocatalytic, and magnetic properties of polycrystalline Zn1−xCoxO (x = 0.01–0.06) samples have been considered. Structural analyses based on powder X-ray diffraction, Rietveld refinement, and Raman spectroscopy prove all materials having the P63mc wurtzite-type structure. The Co-doping and hydrogenation changed the concentration of Zn– and O–related defects whose energy levels occupy the band gap. This also enhanced photocatalytic performance of hydrogenated samples with x > 0.02. X-ray and UV–Vis absorption analyses indicate the substitution of Co2+ for Zn2+ in the wurtzite-type ZnO lattice, leading to irregularly changed the unit-cell parameters. While all the as-prepared samples are paramagnetic, the hydrogenated ones exhibit weak ferromagnetism. Ferromagnetic (FM) ordering increases when x increases, particularly for x ≥ 0.02. According to the results achieved from studying crystalline and electronic structures, we believe that oxygen-vacancies-mediated interactions between Co2+ ions and H–Co–H exchange dimers enhanced FM ordering in hydrogenated Zn1−xCoxO. Computational investigations have also indicated that the magnetization value of H–Zn1−xCoxO is influenced by the positioning of the H dopant, meaning that the couplings between Co and H play an essential role in establishing FM order in H–Zn1−xCoxO.
Graphical Abstract













Similar content being viewed by others
Data availability
All data relevant to this study are presented in this article and are available upon request.
References
Singh P, Kumar R, Singh RK (2019) Progress on transition metal-doped ZnO nanoparticles and its application. Ind Eng Chem Res 58:17130–17163. https://doi.org/10.1021/acs.iecr.9b01561
Paskov PP, Monemar B (2017) Optical properties of III-nitride semiconductors In: Handbook of GaN semiconductor materials and devices. CRC Press, Boca Raton: Taylor & Francis, CRC Press, Series in optics and optoelectronics, pp 87–116
Abdul Amir HAA, Fakhri MA, Abdulkhaleq Alwahib A (2021) Review of GaN optical device characteristics, applications, and optical analysis technology. Mater Today Proc 42:2815–2821. https://doi.org/10.1016/j.matpr.2020.12.727
Khan MAH, Rao MV (2020) Gallium nitride (GaN) nanostructures and their gas sensing properties: a review. Sensors 20:3889. https://doi.org/10.3390/s20143889
Meneghini M, De Santi C, Abid I et al (2021) GaN-based power devices: physics, reliability, and perspectives. J Appl Phys 130:181101. https://doi.org/10.1063/5.0061354
Sharma DK, Shukla S, Sharma KK, Kumar V (2022) A review on ZnO: fundamental properties and applications. Mater Today Proc 49:3028–3035. https://doi.org/10.1016/j.matpr.2020.10.238
Beitollahi H, Tajik S, Garkani Nejad F, Safaei M (2020) Recent advances in ZnO nanostructure-based electrochemical sensors and biosensors. J Mater Chem B 8:5826–5844. https://doi.org/10.1039/D0TB00569J
Cao S, Zheng J, Zhao J et al (2017) Enhancing the performance of quantum dot light-emitting diodes using room-temperature-processed Ga-doped ZnO nanoparticles as the electron transport layer. ACS Appl Mater Interfaces 9:15605–15614. https://doi.org/10.1021/acsami.7b03262
Ong CB, Ng LY, Mohammad AW (2018) A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew Sustain Energy Rev 81:536–551. https://doi.org/10.1016/j.rser.2017.08.020
Dietl T, Ohno H, Matsukura F, Cibert J, Ferrand D (2000) Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science 287:1019–1022. https://doi.org/10.1126/science.287.5455.1019
Sharma P, Gupta A, Rao KV et al (2003) Ferromagnetism above room temperature in bulk and transparent thin films of Mn-doped ZnO. Nat Mater 2:673–677. https://doi.org/10.1038/nmat984
García MA, Ruiz-González ML, Quesada A et al (2005) Interface double-exchange ferromagnetism in the Mn–Zn–O system: new class of biphase magnetism. Phys Rev Lett 94:217206. https://doi.org/10.1103/PhysRevLett.94.217206
Phan T-L, Ho TA, Dang NT et al (2017) Electronic structure, optical and magnetic studies of PLD-grown (Mn, P)-doped ZnO nanocolumns at room temperature. J Phys D Appl Phys 50:295002. https://doi.org/10.1088/1361-6463/aa75e5
Phan T-L, Yu SC (2013) Optical and magnetic properties of Zn1–xMnxO nanorods grown by chemical vapor deposition. J Phys Chem C 117:6443–6453. https://doi.org/10.1021/jp312080v
Kittilstved KR, Liu WK, Gamelin DR (2006) Electronic structure origins of polarity-dependent high-TC ferromagnetism in oxide-diluted magnetic semiconductors. Nat Mater 5:291–297. https://doi.org/10.1038/nmat1616
Pal B, Giri PK (2010) High temperature ferromagnetism and optical properties of Co doped ZnO nanoparticles. J Appl Phys 108:084322. https://doi.org/10.1063/1.3500380
Dhara S, Sundaravel B, Nair KGM et al (2006) Ferromagnetism in cobalt-doped n-GaN. Appl Phys Lett 88:173110. https://doi.org/10.1063/1.2194347
Yu X, Gao Y, Sun S et al (2023) Magnetic and electric properties of Co doped ZnO films via in-situ growth. Phys B Condens Matter 649:414493. https://doi.org/10.1016/j.physb.2022.414493
Li Y, Cao C, Chen Z (2010) Ferromagnetic Fe-doped GaN nanowires grown by chemical vapor deposition. J Phys Chem C 114:21029–21034. https://doi.org/10.1021/jp106256b
Beltrán JJ, Barrero CA, Punnoose A (2015) Understanding the role of iron in the magnetism of Fe doped ZnO nanoparticles. Phys Chem Chem Phys 17:15284–15296. https://doi.org/10.1039/C5CP01408E
Ali N, Singh B, Khan ZA et al (2019) Origin of ferromagnetism in Cu-doped ZnO. Sci Rep 9:2461. https://doi.org/10.1038/s41598-019-39660-x
Seong H-K, Kim J-Y, Kim J-J et al (2007) Room-temperature ferromagnetism in Cu doped GaN nanowires. Nano Lett 7:3366–3371. https://doi.org/10.1021/nl0716552
Haq K, Irfan M, Masood M et al (2018) Enhanced room temperature ferromagnetism in Cr-doped ZnO nanoparticles prepared by auto-combustion method. J Semicond 39:043001. https://doi.org/10.1088/1674-4926/39/4/043001
Liu SH, Hsu HS, Venkataiah G et al (2010) Reduced room-temperature ferromagnetism in intermediate conducting regime of V doped ZnO. Appl Phys Lett 96:262504. https://doi.org/10.1063/1.3456381
Rana AK, Kumar Y, Rajput P et al (2017) Search for origin of room temperature ferromagnetism properties in Ni-doped ZnO nanostructure. ACS Appl Mater Interfaces 9:7691–7700. https://doi.org/10.1021/acsami.6b12616
Dhiman P, Rana G, Kumar A et al (2023) Rare earth doped ZnO nanoparticles as spintronics and photo catalyst for degradation of pollutants. Molecules 28:2838. https://doi.org/10.3390/molecules28062838
Parthasaradi V, Kavitha M, Sridevi A, Rubia JJ (2022) Novel rare-earth Eu and La co-doped ZnO nanoparticles synthesized via co-precipitation method: optical, electrical, and magnetic properties. J Mater Sci Mater Electron 33:25805–25819. https://doi.org/10.1007/s10854-022-09272-9
Shakil M, Hussain A, Zafar M et al (2018) Ferromagnetism in GaN doped with transition metals and rare-earth elements: a review. Chin J Phys 56:1570–1577. https://doi.org/10.1016/j.cjph.2018.05.018
Coey JMD (2005) Ferromagnetism. Solid State Sci 7:660–667. https://doi.org/10.1016/j.solidstatesciences.2004.11.012
Phan T-L, Zhang YD, Yang DS et al (2013) Defect-induced ferromagnetism in ZnO nanoparticles prepared by mechanical milling. Appl Phys Lett 102:072408. https://doi.org/10.1063/1.4793428
Straumal BB, Protasova SG, Mazilkin AA et al (2016) Ferromagnetic behaviour of ZnO: the role of grain boundaries. Beilstein J Nanotechnol 7:1936–1947. https://doi.org/10.3762/bjnano.7.185
Yin X, Wang Y, Jacobs R et al (2019) Massive vacancy concentration yields strong room-temperature ferromagnetism in two-dimensional ZnO. Nano Lett 19:7085–7092. https://doi.org/10.1021/acs.nanolett.9b02581
Wang B, Wang D, Ning J et al (2021) Robust magnetic behavior in two-dimensional GaN caused by atomic vacancies. J Mater Sci 56:2311–2322. https://doi.org/10.1007/s10853-020-05395-8
Coey JMD, Venkatesan M, Fitzgerald CB (2005) Donor impurity band exchange in dilute ferromagnetic oxides. Nat Mater 4:173–179. https://doi.org/10.1038/nmat1310
Jiang Y, Yan W, Sun Z et al (2009) Experimental and theoretical investigations on ferromagnetic nature of Mn-doped dilute magnetic semiconductors. J Phys Conf Ser 190:012100. https://doi.org/10.1088/1742-6596/190/1/012100
Moulahi A (2021) Co addition for improved photocatalytic properties of ZnO nanoparticles. Egypt J Chem 64(11):6147–6154. https://doi.org/10.21608/ejchem.2021.71266.3564
Li S-S, Su Y-K (2019) Conductive filaments controlled ferromagnetism in Co-doped ZnO resistive switching memory device. Jpn J Appl Phys 58:SBBI01. https://doi.org/10.7567/1347-4065/aaf7fb
Kanwal S, Khan MT, Mehboob N et al (2022) Room-temperature ferromagnetism in Cu/Co Co-doped ZnO nanoparticles prepared by the Co-precipitation method: For spintronics applications. ACS Omega 7:32184–32193. https://doi.org/10.1021/acsomega.2c03375
Murtaza A, Zuo W, Song X et al (2022) Robust ferromagnetism in rare-earth and transition metal co-doped ZnO nanoparticles for spintronics applications. Mater Lett 310:131479. https://doi.org/10.1016/j.matlet.2021.131479
Khan R, Althubeiti K, Zulfiqar et al (2021) Structure and magnetic properties of (Co, Ce) co-doped ZnO-based diluted magnetic semiconductor nanoparticles. J Mater Sci Mater Electron 32:24394–24400. https://doi.org/10.1007/s10854-021-06912-4
Jindal K, Tomar M, Gupta V (2014) Stabilization of ferromagnetism in Co codoped ZnO:N. Integr Ferroelectr 158:90–97. https://doi.org/10.1080/10584587.2014.957139
Chen R, Luo F, Liu Y et al (2021) Tunable room-temperature ferromagnetism in Co-doped two-dimensional van der Waals ZnO. Nat Commun 12:3952. https://doi.org/10.1038/s41467-021-24247-w
Chan Cho Y, Lee S, Hun Park J et al (2014) Hydrogen-induced anomalous Hall effect in Co-doped ZnO. New J Phys 16:073030. https://doi.org/10.1088/1367-2630/16/7/073030
Cho YC, Kim S-J, Lee S et al (2009) Reversible ferromagnetic spin ordering governed by hydrogen in Co-doped ZnO semiconductor. Appl Phys Lett 95:172514. https://doi.org/10.1063/1.3257733
de Godoy MPF, Mesquita A, Avansi W et al (2013) Evidence of defect-mediated magnetic coupling on hydrogenated Co-doped ZnO. J Alloys Compd 555:315–319. https://doi.org/10.1016/j.jallcom.2012.11.105
Edelman IS, Chou H, Samoshkina YE et al (2019) Giant hydrogen effect on the structure and physical properties of ZnO and Co-doped ZnO films fabricated by the RF magnetron sputtering in Ar+H2 atmosphere. J Magn Magn Mater 489:165461. https://doi.org/10.1016/j.jmmm.2019.165461
Varvaro G, Di Trolio A, Polimeni A et al (2019) Giant magneto-optical response in H+ irradiated Zn1−xCoxO thin films. J Mater Chem C 7:78–85. https://doi.org/10.1039/C8TC03563F
El-Hilo M, Dakhel AA, Yacoob ZJ (2019) Magnetic interactions in Co2+ doped ZnO synthesised by co-precipitation method: efficient effect of hydrogenation on the long-range ferromagnetic order. J Magn Magn Mater 482:125–134. https://doi.org/10.1016/j.jmmm.2019.03.053
Teo BK (1986) EXAFS: Basic principles and data analysis. Springer, Berlin Heidelberg
Giannozzi P, Baroni S, Bonini N et al (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21:395502. https://doi.org/10.1088/0953-8984/21/39/395502
Giannozzi P, Andreussi O, Brumme T et al (2017) Advanced capabilities for materials modelling with Quantum ESPRESSO. J Phys Condens Matter 29:465901. https://doi.org/10.1088/1361-648X/aa8f79
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Xiao Q, Zhang J, Xiao C, Tan X (2007) Photocatalytic decolorization of methylene blue over Zn1−xCoxO under visible light irradiation. Mater Sci Eng B 142:121–125. https://doi.org/10.1016/j.mseb.2007.06.021
Saadi H, Khaldi O, Pina J et al (2024) Effect of Co doping on the physical properties and organic pollutant photodegradation efficiency of ZnO nanoparticles for environmental applications. Nanomaterials 14:122. https://doi.org/10.3390/nano14010122
Oladoye PO, Bamigboye MO, Ogunbiyi OD, Akano MT (2022) Toxicity and decontamination strategies of Congo red dye. Groundw Sustain Dev 19:100844. https://doi.org/10.1016/j.gsd.2022.100844
Zheng Y, Cao L, Xing G et al (2019) Microscale flower-like magnesium oxide for highly efficient photocatalytic degradation of organic dyes in aqueous solution. RSC Adv 9:7338–7348. https://doi.org/10.1039/C8RA10385B
Devi PG, Velu AS (2016) Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. J Theor Appl Phys 10:233–240. https://doi.org/10.1007/s40094-016-0221-0
Shunmuga Sundaram P, Sangeetha T, Rajakarthihan S et al (2020) XRD structural studies on cobalt doped zinc oxide nanoparticles synthesized by coprecipitation method: Williamson–Hall and size-strain plot approaches. Phys B Condens Matter 595:412342. https://doi.org/10.1016/j.physb.2020.412342
Pessoni HVS, Banerjee P, Franco A (2018) Colossal dielectric permittivity in Co-doped ZnO ceramics prepared by a pressure-less sintering method. Phys Chem Chem Phys 20:28712–28719. https://doi.org/10.1039/C8CP04215B
Birajdar SD, Bhagwat VR, Shinde AB, Jadhav KM (2016) Effect of Co2+ ions on structural, morphological and optical properties of ZnO nanoparticles synthesized by sol–gel auto combustion method. Mater Sci Semicond Process 41:441–449. https://doi.org/10.1016/j.mssp.2015.10.002
Gandhi V, Ganesan R, Abdulrahman Syedahamed HH, Thaiyan M (2014) Effect of cobalt doping on structural, optical, and magnetic properties of ZnO nanoparticles synthesized by coprecipitation method. J Phys Chem C 118:9715–9725. https://doi.org/10.1021/jp411848t
Djerdj I, Garnweitner G, Arčon D et al (2008) Diluted magnetic semiconductors: Mn/Co-doped ZnO nanorods as case study. J Mater Chem 18:5208. https://doi.org/10.1039/b808361d
Gaudon M, Toulemonde O, Demourgues A (2007) Green coloration of Co-doped ZnO explained from structural refinement and bond considerations. Inorg Chem 46:10996–11002. https://doi.org/10.1021/ic701157j
Kumar S, Basu S, Rana B et al (2014) Structural, optical and magnetic properties of sol–gel derived ZnO:Co diluted magnetic semiconductor nanocrystals: an EXAFS study. J Mater Chem C 2:481–495. https://doi.org/10.1039/C3TC31834F
Ji H, Cai C, Zhou S, Liu W (2018) Structure, photoluminescence, and magnetic properties of Co-doped ZnO nanoparticles. J Mater Sci Mater Electron 29:12917–12926. https://doi.org/10.1007/s10854-018-9411-7
Van de Walle CG (2000) Hydrogen as a cause of doping in zinc oxide. Phys Rev Lett 85:1012–1015. https://doi.org/10.1103/PhysRevLett.85.1012
Du M-H, Biswas K (2011) Anionic and hidden hydrogen in ZnO. Phys Rev Lett 106:115502. https://doi.org/10.1103/PhysRevLett.106.115502
Chan KS, Vines L, Johansen KM et al (2013) Defect formation and thermal stability of H in high dose H implanted ZnO. J Appl Phys 114:083111
Beltrán JJ, Barrero CA, Punnoose A (2016) Identifying the sources of ferromagnetism in sol-gel synthesized Zn1−xCoxO (0≤x≤0.10) nanoparticles. J Solid State Chem 240:30–42. https://doi.org/10.1016/j.jssc.2016.05.013
Pessoni HVS, Franco A Jr (2022) Magnetic properties in randomly diluted magnetic systems: Co-doped ZnO polycrystalline ceramics. J Alloys Compd 923:166264. https://doi.org/10.1016/j.jallcom.2022.166264
Phan T-L, Nghia NX, Yu SC (2012) Raman scattering spectra and magnetic properties of polycrystalline Zn1−xCoxO ceramics. Solid State Commun 152:2087–2091. https://doi.org/10.1016/j.ssc.2012.08.026
Parayanthal P, Pollak FH (1984) Raman scattering in alloy semiconductors: “spatial correlation” model. Phys Rev Lett 52:1822–1825. https://doi.org/10.1103/PhysRevLett.52.1822
Phan TL, Vincent R, Cherns D et al (2008) Raman scattering in Me-doped ZnO nanorods (Me=Mn Co, Cu and Ni) prepared by thermal diffusion. Nanotechnology 19:475702. https://doi.org/10.1088/0957-4484/19/47/475702
Gabás M, Landa-Cánovas A, Luis Costa-Krämer J et al (2013) Differences in n-type doping efficiency between Al- and Ga-ZnO films. J Appl Phys 113:163709. https://doi.org/10.1063/1.4803063
Housecroft CE, Sharpe AG (2012) Inorganic chemistry, 4th edn. Pearson, Lincolnshire
Bhat SV, Deepak FL (2005) Tuning the bandgap of ZnO by substitution with Mn2+, Co2+ and Ni2+. Solid State Commun 135:345–347. https://doi.org/10.1016/j.ssc.2005.05.051
K.Sivan A, Galán-González A, Di Mario L et al (2021) Optical properties and carrier dynamics in Co-doped ZnO nanorods. Nanoscale Adv 3:214–222. https://doi.org/10.1039/D0NA00693A
Samavati A, Ismail AF, Nur H et al (2016) Spectral features and antibacterial properties of Cu-doped ZnO nanoparticles prepared by sol-gel method. Chin Phys B 25:077803. https://doi.org/10.1088/1674-1056/25/7/077803
Kuriakose S, Satpati B, Mohapatra S (2014) Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys Chem Chem Phys 16:12741. https://doi.org/10.1039/c4cp01315h
Vempati S, Mitra J, Dawson P (2012) One-step synthesis of ZnO nanosheets: a blue-white fluorophore. Nanoscale Res Lett 7:470. https://doi.org/10.1186/1556-276X-7-470
Zeng H, Duan G, Li Y et al (2010) Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: defect origins and emission controls. Adv Funct Mater 20:561–572. https://doi.org/10.1002/adfm.200901884
Ramasubramanian S, Thangavel R, Rajagopalan M et al (2013) Study on the ferromagnetism in Co and N doped ZnO thin films. Curr Appl Phys 13:1547–1553. https://doi.org/10.1016/j.cap.2013.05.010
Güy N, Özacar M (2016) The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int J Hydrogen Energy 41:20100–20112. https://doi.org/10.1016/j.ijhydene.2016.07.063
Daou I, Zegaoui O, Elghazouani A (2017) Physicochemical and photocatalytic properties of the ZnO particles synthesized by two different methods using three different precursors. Comptes Rendus Chim 20:47–54. https://doi.org/10.1016/j.crci.2016.04.003
Ivill M, Pearton SJ, Rawal S et al (2008) Structure and magnetism of cobalt-doped ZnO thin films. New J Phys 10:065002. https://doi.org/10.1088/1367-2630/10/6/065002
Ciatto G, Di Trolio A, Fonda E et al (2011) Evidence of cobalt-vacancy complexes in Zn1−xCoxO dilute magnetic semiconductors. Phys Rev Lett 107:127206. https://doi.org/10.1103/PhysRevLett.107.127206
Wei H, Yao T, Pan Z et al (2009) Role of Co clusters in wurtzite Co:ZnO dilute magnetic semiconductor thin films. J Appl Phys 105:043903. https://doi.org/10.1063/1.3074297
Robkhob P, Tang IM, Thongmee S (2019) Magnetic properties of the dilute magnetic semiconductor Zn1−xCoxO nanoparticles. J Supercond Nov Magn 32:3637–3645. https://doi.org/10.1007/s10948-019-5135-z
Hao Y, Zhou L, Li J, Hu Z (2016) Structural, morphological and magnetic properties of Zn1−x CoxO prepared by sol-gel and hydrothermal method combined. J Semicond 37:113002. https://doi.org/10.1088/1674-4926/37/11/113002
Singh GP, Aman AK, Singh RK, Roy MK (2020) Effect of low Co-doping on structural, optical, and magnetic performance of ZnO nanoparticles. Optik (Stuttg) 203:163966. https://doi.org/10.1016/j.ijleo.2019.163966
Franco A, Pessoni HVS, Ribeiro PRT, Machado FLA (2017) Magnetic properties of Co-doped ZnO nanoparticles. J Magn Magn Mater 426:347–350. https://doi.org/10.1016/j.jmmm.2016.10.159
Castro TJ, Rodrigues PAM, Oliveira AC et al (2017) Optical and magnetic properties of Co-doped ZnO nanoparticles and the onset of ferromagnetic order. J Appl Phys 121:013904. https://doi.org/10.1063/1.4973526
Chanda A, Gupta S, Vasundhara M et al (2017) Study of structural, optical and magnetic properties of cobalt doped ZnO nanorods. RSC Adv 7:50527–50536. https://doi.org/10.1039/C7RA08458G
Azam A, Ahmed F, Habib SS et al (2013) Fabrication of Co-doped ZnO nanorods for spintronic devices. Met Mater Int 19:845–850. https://doi.org/10.1007/s12540-013-4027-1
Iqbal J, Janjua RA, Jan T (2014) Structural, optical and magnetic properties of Co-doped ZnO nanoparticles prepared via a wet chemical route. Int J Mod Phys B 28:1450158. https://doi.org/10.1142/S0217979214501586
Guruvammal D, Selvaraj S, Meenakshi Sundar S (2018) Structural, optical and magnetic properties of Co doped ZnO DMS nanoparticles by microwave irradiation method. J Magn Magn Mater 452:335–342. https://doi.org/10.1016/j.jmmm.2017.12.097
Wang Q, Sun Q, Jena P (2010) First-principles study of the effect of vacancies on magnetic properties of Zn1−xCoxO thin films. J Phys Condens Matter 22:076002. https://doi.org/10.1088/0953-8984/22/7/076002
MacManus-Driscoll JL, Khare N, Liu Y, Vickers ME (2007) Structural evidence for Zn intersititials in ferromagnetic Zn1–xCoxO films. Adv Mater 19:2925–2929. https://doi.org/10.1002/adma.200602215
Acknowledgements
This work has been supported/partly supported by the VNU University of Engineering and Technology under project number CN23.09.
Author information
Authors and Affiliations
Contributions
T.L.P. conceived and provided supervision for the entire project. D.N.P. carried magnetic measurements, conception, analysis, and review. N.D.C., B.D.T., and N.D.L. carried out sample synthesis, data acquisition, and analysis. N.T.D. and D.T.K. conducted the XRD experiments, Rietveld analysis, review, and editing. T.V.Q, V.Q.N., and J.H.L. carried out theoretical calculations, review, and editing. B.T.H. performed optical investigations, analysis, and review. D.S.Y. caried out the EXAFS measurements, review, and editing. All authors were consulted for discussions on the data and contributed to manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could affect the work reported in this article.
Ethical approval
There are no ethical issues associated with this study and the included experiments.
Additional information
Handling Editor: Pedro Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petrov, D.N., Dang, N.T., Co, N.D. et al. Enhanced photocatalytic activity and ferromagnetic ordering in hydrogenated Zn1−xCoxO. J Mater Sci 59, 9217–9236 (2024). https://doi.org/10.1007/s10853-024-09724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09724-z