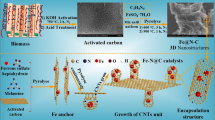
Abstract
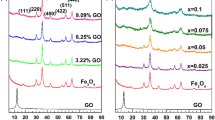
Fe–N co-doped carbon catalyst has drawn increasing attention in the field of Fenton-like degradation of organic wastewater due to their unique structure and high activity. In this study, Fe–N co-doped carbon catalyst with high catalytic efficiency was prepared by one-step pyrolysis modified chitosan with iron ion absorption, and their property was characterized by XRD, BET, XPS and FTIR. The results demonstrated that the Fe–N co-doped carbon catalyst (αFe@CTS-T) has abundant porous structure, surface functional groups, and unique Fe–Nx configuration, which exhibited a superior catalytic ability for degradation of RhB in H2O2 system, and the removal efficiency of RhB was as high as 99.5% within 30min. In addition, further mechanistic insights by contrast and quenching experiments verified that high removal efficient of RhB mainly attribute to indirect oxidation of surface-bound radicals, 1O2 in the H2O2 system, and the pathway of radical generation was proposed.












Similar content being viewed by others
Data and code availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information file.
References
Yan F, Li N, Wang J, Wu H (2023) Ecological footprint model of heavy metal pollution in water environment based on the potential ecological risk index. J Environ Manage 344:118708
Yin H, Qiu P, Qian Y et al (2019) Textile wastewater treatment for water reuse: a case study. Processes 7:34
Nguyen Thi H, Pham Thi H, Nguyen Hoai T, Nguyen Bich N, Vu Ngoc D, Nguyen Thi Bich V (2022) Study on catalytic activity of Co(II) in Rhodamine B decolorization by peroxymonocarbonate in aqueous solution. Vietnam J Chem 60:96
Hirami Y, Hunge YM, Suzuki N et al (2023) Enhanced degradation of ibuprofen using a combined treatment of plasma and Fenton reactions. J Colloid Interface Sci 642:829
Jiang Y, Ran J, Mao K et al (2022) Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments. Ecotoxicol Environ Saf 236:113464
Amruta P, Sahu JN, Anil Kumar P, Prabir G (2023) Current perspective of nano-engineered metal oxide based photocatalysts in advanced oxidation processes for degradation of organic pollutants in wastewater. Chem Eng Res Des 190:667
Yong L, Jianlong W (2023) Multivalent metal catalysts in Fenton/Fenton-like oxidation system: a critical review. Chem Eng J 466:143147
Yang T, Yu D, Wang D et al (2020) Accelerating Fe(III)/Fe(II) cycle via Fe(II) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl Catal B 286:119859
Liu C, Wang P, Huang P, Yang Z, Zhou G (2023) Photo-induced heterogeneous regeneration of Fe(II) in Fenton reaction for efficient polycyclic antibiotics removal and in-depth charge transfer mechanism. J Colloid Interface Sci 638:768
Yang R, Lingjun K, Yiwen Z et al (2021) Review on the synthesis and activity of iron-based catalyst in catalytic oxidation of refractory organic pollutants in wastewater. J Clean Prod 321:128924
Jianhua Q, Lia Z, Bia F et al (2023) A multiple Kirkendall strategy for converting nanosized zero-valent iron to highly active Fenton-like catalyst for organics degradation. Proc Natl Acad Sci 120:e2304552120
Wang G, Nie X, Ji X et al (2018) Enhanced heterogeneous activation of peroxymonosulfate by Co and N codoped porous carbon for degradation of organic pollutants: the synergism between Co and N†. Environ Sci Nano 6:399
Peng X, Wu J, Zhao Z et al (2021) Activation of peroxymonosulfate by single-atom Fe-g-C3N4 catalysts for high efficiency degradation of tetracycline via nonradical pathways: role of high-valent iron-oxo species and Fe–Nx sites. Chem Eng J 427:130803
Jinyuan Z, Lu W, Waseem H et al (2023) The efficient degradation of paracetamol using covalent triazine framework-derived Fe-N-C activated peroxymonosulfate via a non-radical pathway: analysis of high-valent iron oxide, singlet oxygen and electron transfer. Sep Purif Technol 310:123034
Qin L, Chen W, Lai C et al (2023) Highly efficient reduction of nitrophenols by Fe-N-C single-atom catalyst: performance and mechanism insights. J Environ Chem Eng 11:110278
Wang J, Li B, Li Y et al (2021) Facile synthesis of atomic Fe-N-C materials and dual roles investigation of Fe-N4 sites in Fenton-like reactions. Adv Sci 8:2101824
Miao W, Liu Y, Wang D et al (2021) The role of Fe-Nx single-atom catalytic sites in peroxymonosulfate activation: formation of surface-activated complex and non-radical pathways. Chem Eng J 423:130250
Baran T, Karaoğlu K, Nasrollahzadeh M (2022) Nano-sized and microporous palladium catalyst supported on modified chitosan/cigarette butt composite for treatment of environmental contaminants. Environ Res 220:115153
Kazemi Shariat Panahi H, Dehhaghi M, Amiri H et al (2023) Current and emerging applications of saccharide-modified chitosan: a critical review. Biotechnol Adv 66:108172
Dhakshinamoorthy A, Jacob M, Vignesh NS, Varalakshmi P (2020) Pristine and modified chitosan as solid catalysts for catalytic and biodiesel production: a minireview. Int J Biol Macromol 167:807
Liuzhou C, Kun W, Mohe Z et al (2023) Synthesis of carbon disulfide modified chitosan resin and its adsorption properties for palladium(II) in wastewater. Chem Eng J 466:143082
Redouane H, Hamza I, Rahime Eshaghi M et al (2023) Exploring ZnO/montmorillonite photocatalysts for the removal of hazardous RhB dye: a combined study using molecular dynamics simulations andexperiments. Mater Today Commun 35:105915
Yujiao W, Li W, Fang M, Yongqiang Y (2022) FeOx@graphitic carbon core–shell embedded in microporous N-doped biochar activated peroxydisulfate for removal of Bisphenol A: multiple active sites induced non-radical/radical mechanism. Chem Eng J 438:135552
Hongjuan Z, Wei X, Jianping G, Jing T (2021) Hierarchically ordered macro-microporous metal-organic framework derived oxygen reduction electrocatalyst. Chem Eng J 429:132214
Negro E, Videla AHAM, Baglio V, Aricò AS, Specchia S, Koper GJM (2014) Fe–N supported on graphitic carbon nano-networks grown from cobalt as oxygen reduction catalysts for low-temperature fuel cells. Appl Catal B 166–167:75
Daniel G, Kosmala T, Brombin F et al (2021) Highly graphitized Fe-N-C electrocatalysts prepared from chitosan hydrogel frameworks. Catalysts 11:390
Zhang H, Hwang S, Wang M et al (2017) Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J Am Chem Soc 139:14143
Yahui M, Dalin W, Yin X, Heng L, Hui Z (2022) Nonradical electron transfer-based peroxydisulfate activation by a Mn−Fe bimetallic oxide derived from spent alkaline battery for the oxidation of bisphenol A. J Hazard Mater 436:129172
Xiao T, Wang Y, Wan J et al (2021) Fe-N-C catalyst with Fe-NX sites anchored nano carboncubes derived from Fe-Zn-MOFs activate peroxymonosulfate for high-effective degradation of ciprofloxacin: thermal activation and catalytic mechanism. J Hazard Mater 436:129172
Xiangwei Z, Yangyu L, Chunquan L et al (2021) Fast and lasting electron transfer between γ-FeOOH and g-C3N4/kaolinite containing N vacancies for enhanced visible-light-assisted peroxymonosulfate activation. Chem Eng J 429:132374
Peng L, Duan X, Shang Y, Gao B, Xu X (2021) Engineered carbon supported single iron atom sites and iron clusters from Fe-rich enteromorpha for Fenton-like reactions via nonradical pathways. Appl Catal B 287:119963
Cui C, Guo R, Ren E et al (2020) MXene-based rGO/Nb2CTx/Fe3O4 composite for high absorption of electromagnetic wave. Chem Eng J 405:126626
Song Y, He L, Zhang S et al (2021) Novel impedimetric sensing strategy for detecting ochratoxin A based on NH2-MIL-101(Fe) metal-organic framework doped with cobalt phthalocyanine nanoparticles. Food Chem 351:129248
Chen S, Ma L, Du Y, Zhan W, Zhang TC, Du D (2020) Highly efficient degradation of rhodamine B by carbon nanotubes-activated persulfate. Sep Purif Technol 256:117788
Li Z, Shen C, Liu Y et al (2019) Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton. Appl Catal B 260:118204
Lu T, Gao Y, Yang Y et al (2021) Efficient degradation of tetracycline hydrochloride by photocatalytic ozonation over Bi2WO6. Chemosphere 283:131256
Li Z, Wang M, Jin C et al (2019) Synthesis of novel Co3O4 hierarchical porous nanosheets via corn stem and MOF-Co templates for efficient oxytetracycline degradation by peroxymonosulfate activation. Chem Eng J 392:123789
Abdul Hannan A, Nasir R, Rajan Arjan Kalyan H et al (2022) Graphitic carbon nitride engineered α-Fe2O3/rGO heterostructure for visible-light-driven photochemical oxidation of sulfamethoxazole. Chem Eng J 451:138630
Lina Z, Xingwang Z, Lecheng L (2021) The preparation of δ-FeOOH/γ-Al2O3 under mild conditions as an efficient heterogeneous Fenton catalyst for organic pollutants degradation. J Environ Chem Eng 9:106796
Lan Huong N, Xuan Hoan N, Van Nam T et al (2022) Promoted degradation of ofloxacin by ozone integrated with Fenton-like process using iron-containing waste mineral enriched by magnetic composite as heterogeneous catalyst. J Water Process Eng 49:103000
Yu J, Zhu Z, Zhang H, Qiu Y, Yin D (2021) Fe–nitrogen–doped carbon with dual active sites for efficient degradation of aromatic pollutants via peroxymonosulfate activation. Chem Eng J 427:130898
Ruijie X, Kaiheng G, Zhimin A, Ziyi S, Haibao H, Leung DYC (2023) Surface-bound radicals generated from cobalt single-atom catalyst: mechanism of boosting Fenton-like reactions. Chem Eng J 461:141920
Acknowledgements
This research was supported by National Natural Science Foundation of China (NSAF Grant No. U2030207); Science Research Foundation of Yunnan Provincial Education Department (2022J0441); Yunnan Fundamental Research Projects (202201BE070001-018); National College Student Innovation and Entrepreneurship Training Program Project (202310622008); National Natural Science Foundation of China (22106112); Sichuan Province Science and Technology Support Program (2023NSFSC1118); Zigong Provincial Transfer Payment Technology Support Program (2021SZYZF01); Returned Overseas Program of Sichuan University of Science and Engineering (2021RC25); Vanadium and Titanium Resources Utilization Key Laboratory of Sichuan Province (2018FTSZ12, 2018FTSZ18); Opening Project of Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (CSPC202107); Innovation and Entrepreneurship Training Program of Sichuan University of Science and Engineering (S202010622052); The Innovation Fund of Postgraduate, Sichuan University of Science & Engineering (Y2022020).
Author information
Authors and Affiliations
Contributions
Caiyi Jiang involved in conception, supervision, data curation and writing—original draft. Ming Hou involved in methodology and experimental design. Li Yang involved in validation and supervision. Bo Xing involved in conception and supervision. Shenghui Guo involved in conception, and funding acquisition, Guo Yang involved writing—review and editing, supervision. Yi Wang involved in validation and supervision. Dongzheng Wang and Siyang Zhang involved in experimental data collection and collation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, C., Xing, B., Yang, L. et al. Fe–N co-doped carbon derived from one-step pyrolysis modified chitosan for activating H2O2 to degrade organic wastewater. J Mater Sci (2024). https://doi.org/10.1007/s10853-024-09718-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10853-024-09718-x