Abstract
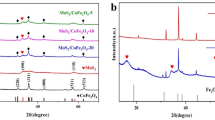
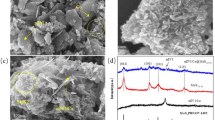
A series of flower-like Fe0-Fe3O4@MoS2 composite catalysts were successfully synthesized by reducing Fe0 on the surface of Fe3O4 hollow spheres and coating with MoS2 to activate peroxomonosulfate (PMS) for tetracycline hydrochloride (TC) degradation. Adding Fe0 increased the Fe2+ content, and the surface-coated 1T/2H mixed phase MoS2 enhanced the redox cycle of Fe (II)/Fe (III) and the reactivity of the heterogeneous system. Subsequently, the quenching experiments showed that radical and non-radical pathways were present in the Fe0-Fe3O4@MoS2/PMS system, and O2−· and 1O2 were dominant in the degradation. Moreover, the experimental results demonstrated that Fe0-Fe3O4@MoS2 exhibited excellent degradation efficiency (96.81%) and anti-interference capability against anions and humic acid. On this basis, the reaction mechanism was analyzed, and three possible degradation pathways were proposed by LC–MS to detect intermediates of TC.







Similar content being viewed by others
Data and code availability
All data are available to readers.
References
Singh R, Singh AP, Kumar S, Giri BS, Kim K-H (2019) Antibiotic resistance in major rivers in the world: a systematic review on occurrence, emergence, and management strategies. J Clean Prod 234:1484–1505. https://doi.org/10.1016/j.jclepro.2019.06.243
Zhu S, Qin L, Li Z, Hu X, Yin D (2023) Effects of nanoplastics and microplastics on the availability of pharmaceuticals and personal care products in aqueous environment. J Hazard Mater 458:131999. https://doi.org/10.1016/j.jhazmat.2023.131999
Kovalakova P, Cizmas L, McDonald TJ, Marsalek B, Feng M, Sharma VK (2020) Occurrence and toxicity of antibiotics in the aquatic environment: a review. Chemosphere 251:126351. https://doi.org/10.1016/j.chemosphere.2020.126351
Shen Q, Wang Z, Yu Q, Cheng Y, Liu Z, Zhang T, Zhou S (2020) Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues. Environ Res 183:109195. https://doi.org/10.1016/j.envres.2020.109195
Selvamani PS, Vijaya JJ, Kennedy LJ, Mustafa A, Bououdina M, Sophia PJ, Ramalingam RJ (2021) Synergic effect of Cu2O/MoS2/rGO for the sonophotocatalytic degradation of tetracycline and ciprofloxacin antibiotics. Ceram Int 47:4226–4237. https://doi.org/10.1016/j.ceramint.2020.09.301
Leichtweis J, Vieira Y, Welter N, Silvestri S, Dotto GL, Carissimi E (2022) A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf Environ Prot 160:25–40. https://doi.org/10.1016/j.psep.2022.01.085
Li J, Cao H, Qi H et al (2023) Efficiently activating peroxymonosulfate by CoFe-LDHs/MoS2 for rapid degradation of tetracycline. J Water Process Eng 53:103856. https://doi.org/10.1016/j.jwpe.2023.103856
Xu L, Zhang H, Xiong P, Zhu Q, Liao C, Jiang G (2021) Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: a review. Sci Total Environ 753:141975. https://doi.org/10.1016/j.scitotenv.2020.141975
Ye Z-X, Shao K-L, Huang H, Yang X (2021) Tetracycline antibiotics as precursors of dichloroacetamide and other disinfection byproducts during chlorination and chloramination. Chemosphere 270:128628. https://doi.org/10.1016/j.chemosphere.2020.128628
Sultan I, Siddiqui MT, Gogry FA, Mohd Q, Haq R (2022) Molecular characterization of resistance determinants and mobile genetic elements of ESBL producing multidrug-resistant bacteria from freshwater lakes in Kashmir, India. Sci Total Environ 827:154221. https://doi.org/10.1016/j.scitotenv.2022.154221
Cai C, Huang X, Dai X (2022) Differential variations of intracellular and extracellular antibiotic resistance genes between treatment units in centralized sewage sludge treatment plants. Water Res 222:118893. https://doi.org/10.1016/j.watres.2022.118893
Wang J, Zhang C, Xiong L, Song G, Liu F (2022) Changes of antibiotic occurrence and hydrochemistry in groundwater under the influence of the South-to-North Water Diversion (the Hutuo River, China). Sci Total Environ 832:154779. https://doi.org/10.1016/j.scitotenv.2022.154779
Wang S, Wang J (2019) Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem Eng J 356:350–358. https://doi.org/10.1016/j.cej.2018.09.062
Dewil R, Mantzavinos D, Poulios I, Rodrigo MA (2017) New perspectives for advanced oxidation processes. J Environ Manag 195:93–99. https://doi.org/10.1016/j.jenvman.2017.04.010
Scaria J, Nidheesh PV (2022) Comparison of hydroxyl-radical-based advanced oxidation processes with sulfate radical-based advanced oxidation processes. Curr Opin Chem Eng 36:100830. https://doi.org/10.1016/j.coche.2022.100830
Liu H, Fu Y, Chen S, Zhang W, Xiang K, Shen F, Xiao R, Chai L, Zhao F (2023) A layered g-C3N4 support single-atom Fe-N4 catalyst derived from hemin to activate PMS for selective degradation of electron-rich compounds via singlet oxygen species. Chem Eng J 474:145571. https://doi.org/10.1016/j.cej.2023.145571
Tang J, Xu J, Zhang H, Liu W, Li H, Xia J, Xing X (2023) High efficient PMS activation by synergistic effects of bimetallic sulfide FeS2@MoS2 for rapid OFX degradation. Chem Eng J 475:146023. https://doi.org/10.1016/j.cej.2023.146023
Zhu S, Li X, Kang J, Duan X, Wang S (2019) Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ Sci Technol 53:307–315. https://doi.org/10.1021/acs.est.8b04669
Zhao C, Shao B, Yan M, Liu Z, Liang Q, He Q, Wu T, Liu Y, Pan Y, Huang J, Wang J, Liang J, Tang L (2021) Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: a review. Chem Eng J 416:128829. https://doi.org/10.1016/j.cej.2021.128829
Liu R, Xu Y, Chen B (2018) Self-assembled nano-FeO(OH)/reduced graphene oxide aerogel as a reusable catalyst for photo-fenton degradation of phenolic organics. Environ Sci Technol 52:7043–7053. https://doi.org/10.1021/acs.est.8b01043
Lan J, Zhang Q, Yang G, Liu Z, Peng F (2023) Removal of tetracycline hydrochloride by N, P co-doped carbon encapsulated Fe2P activating PMS: a non-radical pathway dominated by singlet oxygen. J Environ Chem Eng 11:110481. https://doi.org/10.1016/j.jece.2023.110481
Liu L, Li Y, Li W, Zhong R, Lan Y, Guo J (2020) The efficient degradation of sulfisoxazole by singlet oxygen (1O2) derived from activated peroxymonosulfate (PMS) with Co3O4–SnO2/RSBC. Environ Res 187:109665. https://doi.org/10.1016/j.envres.2020.109665
Lu J, Zhou Y, Zhou Y (2021) Efficiently activate peroxymonosulfate by Fe3O4@MoS2 for rapid degradation of sulfonamides. Chem Eng J 422:130126. https://doi.org/10.1016/j.cej.2021.130126
Jiang F, Qiu B, Sun D (2018) Advanced degradation of refractory pollutants in incineration leachate by UV/Peroxymonosulfate. Chem Eng J 349:338–346. https://doi.org/10.1016/j.cej.2018.05.062
Liu L, Lin S, Zhang W, Farooq U, Shen G, Hu S (2018) Kinetic and mechanistic investigations of the degradation of sulfachloropyridazine in heat-activated persulfate oxidation process. Chem Eng J 346:515–524. https://doi.org/10.1016/j.cej.2018.04.068
Santos A, Fernandez J, Rodriguez S, Dominguez CM, Lominchar MA, Lorenzo D, Romero A (2018) Abatement of chlorinated compounds in groundwater contaminated by HCH wastes using ISCO with alkali activated persulfate. Sci Total Environ 615:1070–1077. https://doi.org/10.1016/j.scitotenv.2017.09.224
Lei Y-J, Tian Y, Fang C, Zhan W, Duan L-C, Zhang J, Zuo W, Kong X-W (2019) Insights into the oxidation kinetics and mechanism of diesel hydrocarbons by ultrasound activated persulfate in a soil system. Chem Eng J 378:122253. https://doi.org/10.1016/j.cej.2019.122253
Yuan G-E, Qin Y, Feng M, Zhang W, Ru X, Zhang X (2021) Synergistic activation of persulfate by natural chalcocite and ferrous ions by promoting the cycling of Fe3+/Fe2+ couple for degradation of organic pollutants. Ecotoxicol Environ Saf 212:111975. https://doi.org/10.1016/j.ecoenv.2021.111975
Luo R, Liu C, Li J, Wang J, Hu X, Sun X, Shen J, Han W, Wang L (2017) Nanostructured CoP: an efficient catalyst for degradation of organic pollutants by activating peroxymonosulfate. J Hazard Mater 329:92–101. https://doi.org/10.1016/j.jhazmat.2017.01.032
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712. https://doi.org/10.1021/es035121o
Wu Y, Zhu J, Bai J, Lin L, Yu C (2023) The ability of pre-magnetized zero-valent iron for peroxymonosulfate activation to remove ofloxacin. Chem Eng J 461:141825. https://doi.org/10.1016/j.cej.2023.141825
Li Z, Li K, Ma S, Dang B, Li Y, Fu H, Du J, Meng Q (2021) Activation of peroxymonosulfate by iron-biochar composites: comparison of nanoscale Fe with single-atom Fe. J Colloid Interface Sci 582:598–609. https://doi.org/10.1016/j.jcis.2020.08.049
Li J, Wan Y, Li Y, Yao G, Lai B (2019) Surface Fe(III)/Fe(II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Appl Catal B Environ 256:117782. https://doi.org/10.1016/j.apcatb.2019.117782
Liu J, Yang Q, Wang D, Li X, Zhong Y, Li X, Deng Y, Wang L, Yi K, Zeng G (2016) Enhanced dewaterability of waste activated sludge by Fe (II)-activated peroxymonosulfate oxidation. Bioresour Technol 206:134–140. https://doi.org/10.1016/j.biortech.2016.01.088
Zhou Z, Ye G, Zong Y, Zhao Z, Xu L, Wu D (2023) Enhanced organic contaminants degradation via coupling molybdenum powder with tripolyphosphate in Fe(II)-based peroxymonosulfate activation. Chem Eng J 471:144512. https://doi.org/10.1016/j.cej.2023.144512
Zhao Y, Lu D, Xu C, Zhong J, Chen M, Xu S, Cao Y, Zhao Q, Yang M, Ma J (2020) Synergistic oxidation - filtration process analysis of catalytic CuFe2O4 - tailored ceramic membrane filtration via peroxymonosulfate activation for humic acid treatment. Water Res 171:115387. https://doi.org/10.1016/j.watres.2019.115387
Wu Y, Chen X, Han Y, Yue D, Cao X, Zhao Y, Qian X (2019) Highly efficient utilization of nano-Fe (0) embedded in mesoporous carbon for activation of peroxydisulfate. Environ Sci Technol 53:9081–9090. https://doi.org/10.1021/acs.est.9b02170
Chen J, Wan J, Li C, Wei Y, Shi H (2022) Synthesis of novel Fe0-Fe3O4/CeO2/C composite cathode for efficient heterogeneous electro-Fenton degradation of ceftriaxone sodium. J Hazard Mater 437:129393. https://doi.org/10.1016/j.jhazmat.2022.129393
Dong C, Wang Z, Ye Z, He J, Zheng Z, Gong X, Zhang J, Lo IMC (2021) Superoxide radicals dominated visible light driven peroxymonosulfate activation using molybdenum selenide (MoSe2) for boosting catalytic degradation of pharmaceuticals and personal care products. Appl Catal B Environ 296:120223. https://doi.org/10.1016/j.apcatb.2021.120223
Du M, Yi Q, Ji J, Zhu Q, Duan H, Xing M, Zhang J (2020) Sustainable activation of peroxymonosulfate by the Mo (IV) in MoS2 for the remediation of aromatic organic pollutants. Chin Chem Lett 31:2803–2808. https://doi.org/10.1016/j.cclet.2020.08.002
Peng X, Yang Z, Hu F, Tan C, Pan Q, Dai H (2022) Mechanistic investigation of rapid catalytic degradation of tetracycline using CoFe2O4@MoS2 by activation of peroxymonosulfate. Sep Purif Technol 287:120525. https://doi.org/10.1016/j.seppur.2022.120525
Li Z, Fan R, Hu Z, Li W, Zhou H, Kang S, Zhang Y, Zhang H, Wang G (2020) Ethanol introduced synthesis of ultrastable 1T-MoS2 for removal of Cr (VI). J Hazard Mater 394:122525. https://doi.org/10.1016/j.jhazmat.2020.122525
Xing M, Xu W, Dong C, Bai Y, Zeng J, Zhou Y, Zhang J, Yin Y (2018) Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem 4:1359–1372. https://doi.org/10.1016/j.chempr.2018.03.002
Xiao C, Hu Y, Li Q, Liu J, Li X, Shi Y, Chen Y, Cheng J (2023) Carbon-doped defect MoS2 co-catalytic Fe3+/peroxymonosulfate process for efficient sulfadiazine degradation: accelerating Fe3+/Fe2+ cycle and 1O2 dominated oxidation. Sci Total Environ 858:159587. https://doi.org/10.1016/j.scitotenv.2022.159587
Chen Y, Zhang G, Liu H, Qu J (2019) Confining free radicals in close vicinity to contaminants enables ultrafast fenton-like processes in the interspacing of MoS2 membranes. Angew Chem Int Ed 58:8134–8138. https://doi.org/10.1002/anie.201903531
Zhu L, Ji J, Liu J, Mine S, Matsuoka M, Zhang J, Xing M (2020) Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control. Angew Chem Int Ed 59:13968–13976. https://doi.org/10.1002/anie.202006059
Yan Z, He F, Zhang J, Fang J, Wang J, Zhou H (2022) Enhanced Fe(III)/PMS system by flower-like MoS2 nanosheet for rapid degradation of tetracycline. J Environ Chem Eng 10:108860. https://doi.org/10.1016/j.jece.2022.108860
Tong M, Liu F, Dong Q, Ma Z, Liu W (2020) Magnetic Fe3O4-deposited flower-like MoS2 nanocomposites for the Fenton-like Escherichia coli disinfection and diclofenac degradation. J Hazard Mater 385:121604. https://doi.org/10.1016/j.jhazmat.2019.121604
Xu J, Chen J, Zhong Y (2023) Ultrathin MoS2 nanosheet-wrapped Fe3O4 nanocrystals synergistically activate peroxymonosulfate for enhanced removal of organic pollutants. Colloids Surf Physicochem Eng Asp 671:131599. https://doi.org/10.1016/j.colsurfa.2023.131599
Shu X, Zhou J, Lian W, Jiang Y, Wang Y, Shu R, Liu Y, Han J, Zhuang Y (2021) Size-morphology control, surface reaction mechanism and excellent electromagnetic wave absorption characteristics of Fe3O4 hollow spheres. J Alloys Compd 854:157087. https://doi.org/10.1016/j.jallcom.2020.157087
Li S, Tang J, Liu Q, Liu X, Gao B (2020) A novel stabilized carbon-coated nZVI as heterogeneous persulfate catalyst for enhanced degradation of 4-chlorophenol. Environ Int 138:105639. https://doi.org/10.1016/j.envint.2020.105639
Fan L, Gong Y, Wan J, Wei Y, Shi H, Liu C (2022) Flower-like molybdenum disulfide decorated ZIF-8-derived nitrogen-doped dodecahedral carbon for electro-catalytic degradation of phenol. Chemosphere 298:134315. https://doi.org/10.1016/j.chemosphere.2022.134315
Gao B, Du X, Ma Y, Li Y, Li Y, Ding S, Song Z, Xiao C (2020) 3D flower-like defected MoS2 magnetron-sputtered on candle soot for enhanced hydrogen evolution reaction. Appl Catal B Environ 263:117750. https://doi.org/10.1016/j.apcatb.2019.117750
Garcia-Segura S, Brillas E (2017) Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J Photochem Photobiol C Photochem Rev 31:1–35. https://doi.org/10.1016/j.jphotochemrev.2017.01.005
Hou D, Zhou W, Liu X, Zhou K, Xie J, Li G, Chen S (2015) Pt nanoparticles/MoS2 nanosheets/carbon fibers as efficient catalyst for the hydrogen evolution reaction. Electrochim Acta 166:26–31. https://doi.org/10.1016/j.electacta.2015.03.067
Shi Y, Han Z, Yang J, Meng Q (2021) Influence of the Hollowness and Size Distribution on the Magnetic Properties of Fe3O4 Nanospheres. Langmuir 37:9605–9612. https://doi.org/10.1021/acs.langmuir.1c01498
Wang X, Zhu T, Chang S, Lu Y, Mi W, Wang W (2020) 3D Nest-Like Architecture of Core-Shell CoFe2O4 @1T/2H-MoS2 Composites with Tunable Microwave Absorption Performance. ACS Appl Mater Interfaces 12:11252–11264. https://doi.org/10.1021/acsami.9b23489
Zhou Y, Zhou L, Zhou Y, Xing M, Zhang J (2020) Z-scheme photo-Fenton system for efficiency synchronous oxidation of organic contaminants and reduction of metal ions. Appl Catal B Environ 279:119365. https://doi.org/10.1016/j.apcatb.2020.119365
Wang Y, Sun H, Duan X, Ang HM, Tadé MO, Wang S (2015) A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol. Appl Catal B Environ 172–173:73–81. https://doi.org/10.1016/j.apcatb.2015.02.016
Lv X, Xu J, Jiang G, Tang J, Xu X (2012) Highly active nanoscale zero-valent iron (nZVI)–Fe3O4 nanocomposites for the removal of chromium (VI) from aqueous solutions. J Colloid Interface Sci 369:460–469. https://doi.org/10.1016/j.jcis.2011.11.049
Lu J, Zhou Y, Lei J, Ao Z, Zhou Y (2020) Fe3O4/graphene aerogels: a stable and efficient persulfate activator for the rapid degradation of malachite green. Chemosphere 251:126402. https://doi.org/10.1016/j.chemosphere.2020.126402
Zhu T, Shen W, Wang X, Song Y-F, Wang W (2019) Paramagnetic CoS2@MoS2 core-shell composites coated by reduced graphene oxide as broadband and tunable high-performance microwave absorbers. Chem Eng J 378:122159. https://doi.org/10.1016/j.cej.2019.122159
Bai R, Yan W, Xiao Y, Wang S, Tian X, Li J, Xiao X, Lu X, Zhao F (2020) Acceleration of peroxymonosulfate decomposition by a magnetic MoS2/CuFe2O4 heterogeneous catalyst for rapid degradation of fluoxetine. Chem Eng J 397:125501. https://doi.org/10.1016/j.cej.2020.125501
Zhu K, Qin W, Gan Y, Huang Y, Jiang Z, Chen Y, Li X, Yan K (2023) Acceleration of Fe3+/Fe2+ cycle in garland-like MIL-101(Fe)/MoS2 nanosheets to promote peroxymonosulfate activation for sulfamethoxazole degradation. Chem Eng J 470:144190. https://doi.org/10.1016/j.cej.2023.144190
Wang Q, Lu J, Yu M, Li H, Lin X, Nie J, Lan N, Wang Z (2023) Sulfur vacancy rich MoS2/FeMoO4 composites derived from MIL-53(Fe) as PMS activator for efficient elimination of dye: Nonradical 1O2 dominated mechanism. Environ Pollut 333:121990. https://doi.org/10.1016/j.envpol.2023.121990
Li Y, Zhu C, Chen L, Liu L, Zhang J, Yang N, Li Y (2023) Self-driven degradation of TC-HCl by CuFe2O4/Bi2O3 activated peroxymonosulfate. Chem Eng J 473:145282. https://doi.org/10.1016/j.cej.2023.145282
Huang W, Jin X, Li Q, Wang Y, Huang D, Fan S, Yan J, Huang Y, Astruc D, Liu X (2023) Co3O4 Nanocubes for Degradation of Oxytetracycline in Wastewater via Peroxymonosulfate Activation. ACS Appl Nano Mater 6:12497–12506. https://doi.org/10.1021/acsanm.3c02260
Yu J, Afzal S, Zeng T, Wang H, Fu H (2023) Degradation of bisphenol A by peroxymonosulfate activated with MIL-88B(Fe) derived CC-Fe/C catalysts: Effect of annealing temperature, performance and mechanism. Catal Commun 177:106660. https://doi.org/10.1016/j.catcom.2023.106660
Zhu S, Xu Y, Zhu Z, Liu Z, Wang W (2020) Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation. Chem Eng J 384:123298. https://doi.org/10.1016/j.cej.2019.123298
Cai W-W, Peng T, Yang B, Xu C, Liu Y-S, Zhao J-L, Gu F-L, Ying G-G (2020) Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: experimental and theoretical studies. Chem Eng J 402:126224. https://doi.org/10.1016/j.cej.2020.126224
Du W, Zhang Q, Shang Y, Wang W, Li Q, Yue Q, Gao B, Xu X (2020) Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation. Appl Catal B Environ 262:118302. https://doi.org/10.1016/j.apcatb.2019.118302
Yang J-CE, Yuan B, Cui H-J, Wang S, Fu M-L (2017) Modulating oxone-MnOx/silica catalytic systems towards ibuprofen degradation: Insights into system effects, reaction kinetics and mechanisms. Appl Catal B Environ 205:327–339. https://doi.org/10.1016/j.apcatb.2016.12.046
Ghanbari F, Moradi M, Gohari F (2016) Degradation of 2,4,6-trichlorophenol in aqueous solutions using peroxymonosulfate/activated carbon/UV process via sulfate and hydroxyl radicals. J Water Process Eng 9:22–28. https://doi.org/10.1016/j.jwpe.2015.11.011
Gao Y, Zou D (2020) Efficient degradation of levofloxacin by a microwave–3D ZnCo2O4/activated persulfate process: Effects, degradation intermediates, and acute toxicity. Chem Eng J 393:124795. https://doi.org/10.1016/j.cej.2020.124795
Zhang R, Sun P, Boyer TH, Zhao L, Huang C-H (2015) Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ Sci Technol 49:3056–3066. https://doi.org/10.1021/es504799n
Chen L, Yang S, Zuo X, Huang Y, Cai T, Ding D (2018) Biochar modification significantly promotes the activity of Co3O4 towards heterogeneous activation of peroxymonosulfate. Chem Eng J 354:856–865. https://doi.org/10.1016/j.cej.2018.08.098
Li W, Liu B, Wang Z, Wang K, Lan Y, Zhou L (2020) Efficient activation of peroxydisulfate (PDS) by rice straw biochar modified by copper oxide (RSBC-CuO) for the degradation of phenacetin (PNT) Chem. Eng J 395:125094. https://doi.org/10.1016/j.cej.2020.125094
Ding D, Yang S, Chen L, Cai T (2020) Degradation of norfloxacin by CoFe alloy nanoparticles encapsulated in nitrogen doped graphitic carbon (CoFe@N-GC) activated peroxymonosulfate. Chem Eng J 392:123725. https://doi.org/10.1016/j.cej.2019.123725
Luo J, Bo S, Qin Y, An Q, Xiao Z, Zhai S (2020) Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation. Chem Eng J 395:125063. https://doi.org/10.1016/j.cej.2020.125063
Peng G, Zhang M, Deng S, Shan D, He Q, Yu G (2018) Adsorption and catalytic oxidation of pharmaceuticals by nitrogen-doped reduced graphene oxide/Fe3O4 nanocomposite. Chem Eng J 341:361–370. https://doi.org/10.1016/j.cej.2018.02.064
Wang L, Zheng X, Yan L, Song W, Li Y, Wu B, Li X (2023) Multiphasic MoS2 activates peroxymonosulfate for efficient removal of oxytetracycline: the dominant role of surface reactive species. Sep Purif Technol 317:123907. https://doi.org/10.1016/j.seppur.2023.123907
Xu J, Chen J, Zhong Y, Cao L, Zhang X, Wang Z, Chen J, Lin S, Xu Q, Chen Y, Yu L (2023) Ultrathin MoS2 nanosheet-wrapped Fe3O4 nanocrystals synergistically activate peroxymonosulfate for enhanced removal of organic pollutants. Colloids Surf Physicochem Eng Asp 671:131599. https://doi.org/10.1016/j.colsurfa.2023.131599
Zhang Y, Zhou J, Chen X, Wang L, Cai W (2019) Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: synergistic effect and degradation pathway. Chem Eng J 369:745–757. https://doi.org/10.1016/j.cej.2019.03.108
Chen Q, Yang W, Zhu J, Fu L, Li D, Zhou L (2020) Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2-x nanotubes heterostructure for degradation of tetracycline hydrochloride. J Hazard Mater 384:121275. https://doi.org/10.1016/j.jhazmat.2019.121275
Liu S-S, Xing Q-J, Chen Y, Zhu M, Jiang X-H, Wu S-H, Dai W, Zou J-P (2019) Photoelectrochemical degradation of organic pollutants using BiOBr anode coupled with simultaneous CO2 reduction to liquid fuels via CuO cathode. ACS Sustain Chem Eng 7:1250–1259. https://doi.org/10.1021/acssuschemeng.8b04917
Wu W, Sun Y, Zhou H (2022) In-situ construction of β-Bi2O3/Ag2O photocatalyst from deactivated AgBiO3 for tetracycline degradation under visible light. Chem Eng J 432:134316. https://doi.org/10.1016/j.cej.2021.134316
Zhu M, Tang Y, Chen X, Liao B, Yu Y, Hou S, Fan X (2022) Internal electric field and oxygen vacancies synergistically optimized Ba2+ doped SrBi2B2O7 for photocatalytic tetracycline degradation from water. Chem Eng J 433:134580. https://doi.org/10.1016/j.cej.2022.134580
Wang A, Zheng Z, Wang H, Chen Y, Luo C, Liang D, Hu B, Qiu R, Yan K (2020) 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl Catal B Environ 277:119171. https://doi.org/10.1016/j.apcatb.2020.119171
Chen D, Bai Q, Ma T, Jing X, Tian Y, Zhao R, Zhu G (2022) Stable metal–organic framework fixing within zeolite beads for effectively static and continuous flow degradation of tetracycline by peroxymonosulfate activation. Chem Eng J 435:134916. https://doi.org/10.1016/j.cej.2022.134916
Yang T, Ma T, Yang L, Dai W, Zhang S, Luo S (2021) A self-supporting UiO-66 photocatalyst with Pd nanoparticles for efficient degradation of tetracycline. Appl Surf Sci 544:148928. https://doi.org/10.1016/j.apsusc.2021.148928
Wu S, Li X, Tian Y, Lin Y, Hu YH (2021) Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light. Chem Eng J 406:126747. https://doi.org/10.1016/j.cej.2020.126747
Li Y, Yu B, Hu Z, Wang H (2022) Construction of direct Z-scheme SnS2@ZnIn2S4@kaolinite heterostructure photocatalyst for efficient photocatalytic degradation of tetracycline hydrochloride. Chem Eng J 429:132105. https://doi.org/10.1016/j.cej.2021.132105
Liu C, Dai H, Tan C, Pan Q, Hu F, Peng X (2022) Photo-Fenton degradation of tetracycline over Z-scheme Fe-g-C3N4/Bi2WO6 heterojunctions: mechanism insight, degradation pathways and DFT calculation. Appl Catal B Environ 310:121326. https://doi.org/10.1016/j.apcatb.2022.121326
Pi Z, Li X, Wang D, Xu Q, Tao Z, Huang X, Yao F, Wu Y, He L, Yang Q (2019) Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: Performance and degradation pathway. J Clean Prod 235:1103–1115. https://doi.org/10.1016/j.jclepro.2019.07.037
Yi X-H, Wang T-Y, Chu H-Y (2022) Effective elimination of tetracycline antibiotics via photoactivated SR-AOP over vivianite: a new application approach of phosphorus recovery product from WWTP. Chem Eng J 449:137784. https://doi.org/10.1016/j.cej.2022.137784
Acknowledgements
The authors would like to acknowledge the funding from the Natural Science Foundation of Heilongjiang Province of China (LH2022B018) and the Youth Science and Technology Innovation Team Project of Heilongjiang Province (2021-KYYWF-0030).
Author information
Authors and Affiliations
Contributions
Pan Liu: Conceptualization, Methodology, Validation, Data curation, Writing-original draft, Writing-review and editing. Haolin Shi: Methodology, Resources, Data curation. Xinyue Feng: Conceptualization, Investigation. Chuntao Liu: Project administration. Fangwei Ma: Resources, Conceptualization. Jiafeng Wan: Supervision, Methodology, Writing-review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This study did not use human or animal tissues.
Additional information
Handling Editor: Dale Huber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, P., Shi, H., Feng, X. et al. Flower-like Fe0-Fe3O4@MoS2 hollow spheres activate peroxymonosulfate for the degradation of tetracycline hydrochloride. J Mater Sci (2024). https://doi.org/10.1007/s10853-024-09697-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10853-024-09697-z