Abstract
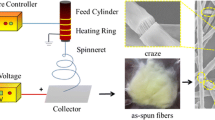
Oleanolic acid (OA) loaded poly(lactic-co-glycolic) acid fiber membranes were developed utilizing the Forcespinning technology. OA is a natural pentacyclic triterpenoid compound available in fruits and vegetables and known for its plethora of biological activities. The incorporation of OA into polymeric fine fiber membranes opens promising potential applications for biomedical applications, such as a system for transdermal delivery of bioactive agents. In this study, nonwoven fiber membranes were developed with different concentrations of OA, and morphological, thermo-physical, and biological studies were conducted. Results show a high yield of fiber membranes with average fiber diameters ranging from 541 to 630 nm, depending on the concentration of OA. Developed membranes are composed of long and continuous fibers showing rough surfaces with stability in aqueous media. Thermo-physical analysis showed miscibility of the components and negligible effects of processing conditions on the structure and stability of the components. High drug loading efficiency (> 80%) was observed, and cellular studies indicated that the developed fiber membranes were not toxic to fibroblast cells. The structural and thermal stability and non-cytotoxic behavior of these membranes make them a promising potential vehicle for drug delivery applications.











Similar content being viewed by others
Change history
16 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10853-023-08350-5
References
Meng ZX, Xu XX, Zheng W, Zhou HM, Li L, Zheng YF, Lou X (2011) Preparation and characterization of electrospun PLGA/gelatin nanofibers as a potential drug delivery system. Coll Surf B Biointerfaces 84:97–102. https://doi.org/10.1016/j.colsurfb.2010.12.022
Malik R, Garg T, Goyal AK, Rath G (2015) Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target 23:109–124. https://doi.org/10.3109/1061186X.2014.965715
Mir M, Ahmed N, ur Rehman A (2017) Recent applications of PLGA based nanostructures in drug delivery. Coll Surf B Biointerfaces 159:217–231. https://doi.org/10.1016/j.colsurfb.2017.07.038
Yang J, liu J, liuhongyan XYZ, lipingchu Q, liu N, cuihong S (2014) Tianjin peptide nanofiber as a carrier for effective curcumin delivery. Int J Nanomed 9:197–207
Mondal K, Ali MA, Agrawal VV, Malhotra BD, Sharma A (2014) Highly sensitive biofunctionalized mesoporous electrospun TiO2 nanofiber based interface for biosensing. ACS Appl Mater Interfaces 6:2516–2527. https://doi.org/10.1021/am404931f
Janković B, Pelipenko J, Škarabot M, Muševič I, Kristl J (2013) The design trend in tissue-engineering scaffolds based on nanomechanical properties of individual electrospun nanofibers. Int J Pharm 455:338–347. https://doi.org/10.1016/j.ijpharm.2013.06.083
Han D, Filocamo S, Kirby R, Steckl AJ (2011) Deactivating chemical agents using enzyme-coated nanofibers formed by electrospinning. ACS Appl Mater Interfaces 3:4633–4639. https://doi.org/10.1021/am201064b
Peng H, Zhou S, Guo T, Li Y, Li X, Wang J, Weng J (2008) In vitro degradation and release profiles for electrospun polymeric fibers containing paracetanol. Coll Surf B Biointerfaces 66:206–212. https://doi.org/10.1016/j.colsurfb.2008.06.021
Shoba E, Lakra R, Kiran MS, Korrapati PS (2017) Fabrication of core-shell nanofibers for controlled delivery of bromelain and salvianolic acid B for skin regeneration in wound therapeutics. Biomed Mater. https://doi.org/10.1088/1748-605X/aa6684
Moydeen AM, Ali Padusha MS, Aboelfetoh EF, Al-Deyab SS, El-Newehy MH (2018) Fabrication of electrospun poly(vinyl alcohol)/dextran nanofibers via emulsion process as drug delivery system: kinetics and in vitro release study. Int J Biol Macromol 116:1250–1259. https://doi.org/10.1016/j.ijbiomac.2018.05.130
Sarkar K, Gomez C, Zambrano S, Ramirez M, De Hoyos E, Vasquez H, Lozano K (2010) Electrospinning to forcespinning™. Mater Today 13:12–14. https://doi.org/10.1016/S1369-7021(10)70199-1
Padron S, Fuentes A, Caruntu D, Lozano K (2013) Experimental study of nanofiber production through forcespinning. J Appl Phys. https://doi.org/10.1063/1.4769886
Zhang X, Tang K, Zheng X (2016) Electrospinning and crosslinking of COL/PVA nanofiber-microsphere containing salicylic acid for drug delivery. J Bionic Eng 13:143–149. https://doi.org/10.1016/S1672-6529(14)60168-2
Eskitoros-Togay M, Bulbul YE, Tort S, Demirtaş Korkmaz F, Acartürk F, Dilsiz N (2019) Fabrication of doxycycline-loaded electrospun PCL/PEO membranes for a potential drug delivery system. Int J Pharm 565:83–94. https://doi.org/10.1016/j.ijpharm.2019.04.073
Khampieng T, Wnek GE, Supaphol P (2014) Electrospun DOXY-h loaded-poly(acrylic acid) nanofiber mats: in vitro drug release and antibacterial properties investigation. J Biomater Sci Polym Ed 25:1292–1305. https://doi.org/10.1080/09205063.2014.929431
Gouda M, Hebeish AA, Aljafari AI (2014) Synthesis and characterization of novel drug delivery system based on cellulose acetate electrospun nanofiber mats. J Ind Text 43:319–329. https://doi.org/10.1177/1528083713495250
Parwe SP, Chaudhari PN, Mohite KK, Selukar BS, Nande SS, Garnaik B (2014) Synthesis of ciprofloxacin-conjugated poly (L-lactic acid) polymer for nanofiber fabrication and antibacterial evaluation. Int J Nanomed 9:1463–1477. https://doi.org/10.2147/IJN.S54971
Almajhdi FN, Fouad H, Khalil KA, Awad HM, Mohamed SHS, Elsarnagawy T, Albarrag AM, Al-Jassir FF, Abdo HS (2014) In-vitro anticancer and antimicrobial activities of PLGA/silver nanofiber composites prepared by electrospinning. J Mater Sci Mater Med 25:1045–1053. https://doi.org/10.1007/s10856-013-5131-y
Semnani K, Shams-Ghahfarokhi M, Afrashi M, Fakhrali A, Semnani D (2018) Antifungal activity of eugenol loaded electrospun PAN nanofiber mats against candida albicans. Curr Drug Deliv 15:860–866. https://doi.org/10.2174/1567201815666180226120436
Akduman C, Özgüney I, Kumbasar EPA (2016) Preparation and characterization of naproxen-loaded electrospun thermoplastic polyurethane nanofibers as a drug delivery system. Mater Sci Eng C 64:383–390. https://doi.org/10.1016/j.msec.2016.04.005
Gentile P, Chiono V, Carmagnola I, Hatton PV (2014) An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci 15:3640–3659. https://doi.org/10.3390/ijms15033640
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–522. https://doi.org/10.1016/j.jconrel.2012.01.043
Guimarães PPG, Oliveira MF, Gomes ADM, Gontijo SML, Cortés ME, Campos PP, Viana CTR, Andrade SP, Sinisterra RD (2015) PLGA nanofibers improves the antitumoral effect of daunorubicin. Coll Surf B Biointerfaces 136:248–255. https://doi.org/10.1016/j.colsurfb.2015.09.005
Shanmugam MK, Dai X, Kumar AP, Tan BKH, Sethi G, Bishayee A (2014) Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: preclinical and clinical evidence. Cancer Lett 346:206–216. https://doi.org/10.1016/j.canlet.2014.01.016
Jie L (1995) Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 49:57–68. https://doi.org/10.1016/0378-8741(95)01310-5
Pollier J, Goossens A (2012) Oleanolic acid. Phytochemistry 77:10–15. https://doi.org/10.1016/j.phytochem.2011.12.022
Khwaza V, Oyedeji OO, Aderibigbe BA (2018) Antiviral activities of oleanolic acid and its analogues. Molecules. https://doi.org/10.3390/molecules23092300
Fukumura M, Ando H, Hirai Y, Toriizuka K, Ida Y, Kuchino Y (2009) Achyranthoside H methyl ester, a novel oleanolic acid saponin derivative from achyranthes fauriei roots, induces apoptosis in human breast cancer MCF-7 and MDA-MB-453 cells via a caspase activation pathway. J Nat Med 63:181–188. https://doi.org/10.1007/s11418-008-0311-7
Tian T, Liu X, Lee ES, Sun J, Feng Z, Zhao L, Zhao C (2017) Synthesis of novel oleanolic acid and ursolic acid in C-28 position derivatives as potential anticancer agents. Arch Pharm Res 40:458–468. https://doi.org/10.1007/s12272-016-0868-8
Dharmappa KK, Kumar RV, Nataraju A, Mohamed R, Shivaprasad HV, Vishwanath BS (2009) Anti-inflammatory activity of oleanolic acid by inhibition of secretory phospholipase A2. Planta Med 75:211–215. https://doi.org/10.1055/s-0028-1088374
Wang X, Ye X, Liu R, Chen HL, Bai H, Liang X, Zhang XDi, Wang Z, Li Wl, Hai CX (2010) Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem Biol Interact 184:328–337. https://doi.org/10.1016/j.cbi.2010.01.034
Zhao H, Zhou M, Duan L, Wang W, Zhang J, Wang D, Liang X (2013) Efficient synthesis and anti-fungal activity of oleanolic acid oxime esters. Molecules 18:3615–3629. https://doi.org/10.3390/molecules18033615
Kong L, Li S, Liao Q, Zhang Y, Sun R, Zhu X, Zhang Q, Wang J, Wu X, Fang X et al (2013) Oleanolic acid and ursolic acid: novel hepatitis C virus antivirals that inhibit NS5B activity. Antiviral Res 98:44–53. https://doi.org/10.1016/j.antiviral.2013.02.003
Mengoni F, Lichtner M, Battinelli L, Marzi M, Mastroianni CM, Vullo V, Mazzanti G (2002) In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med 68:111–114. https://doi.org/10.1055/s-2002-20256
Gao D, Li Q, Li Y, Liu Z, Liu Z, Fan Y, Han Z, Li J, Li K (2007) Antidiabetic potential of oleanolic acid from Ligustrum lucidum Ait. Can J Physiol Pharmacol 85:1076–1083. https://doi.org/10.1139/Y07-098
Wang X, Liu R, Zhang W, Zhang X, Liao N, Wang Z, Li W, Qin X, Hai C (2013) Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol Cell Endocrinol 376:70–80. https://doi.org/10.1016/j.mce.2013.06.014
Liu Q, Liu H, Zhang L, Guo T, Wang P, Geng M, Li Y (2013) Synthesis and antitumor activities of naturally occurring oleanolic acid triterpenoid saponins and their derivatives. Eur J Med Chem 64:1–15. https://doi.org/10.1016/j.ejmech.2013.04.016
Kim S, Lee H, Lee S, Yoon Y, Choi KH (2015) Antimicrobial action of oleanolic acid on listeria monocytogenes, enterococcus faecium, and enterococcus faecalis. PLoS ONE 10:1–11. https://doi.org/10.1371/journal.pone.0118800
Wang ZH, Hsu CC, Huang CN, Yin MC (2010) Anti-glycative effects of oleanolic acid and ursolic acid in kidney of diabetic mice. Eur J Pharmacol 628:255–260. https://doi.org/10.1016/j.ejphar.2009.11.019
Wang Y, Zhu P, Li G, Zhu S, Liu K, Liu Y, He J, Lei J (2019) Amphiphilic carboxylated cellulose-g-poly(L-lactide) copolymer nanoparticles for oleanolic acid delivery. Carbohydr Polym 214:100–109. https://doi.org/10.1016/j.carbpol.2019.03.033
Fu H, Yen FL, Huang PH, Yang CY, Yen CH (2021) Oleanolic acid nanofibers attenuated particulate matter-induced oxidative stress in keratinocytes. Antioxidants 10:1–17. https://doi.org/10.3390/antiox10091411
Fan JP, Zhong H, Zhang XH, Yuan TT, Chen HP, Peng HL (2021) Preparation and characterization of oleanolic acid-based Low-molecular-weight supramolecular hydrogels induced by heating. ACS Appl Mater Interfaces 13:29130–29136. https://doi.org/10.1021/acsami.1c05800
Gao M, Xu H, Bao X, Zhang C, Guan X, Liu H, Lv L, Deng S, Gao D, Wang C et al (2016) Oleanolic acid-loaded PLGA-TPGS nanoparticles combined with heparin sodium-loaded PLGA-TPGS nanoparticles for enhancing chemotherapy to liver cancer. Life Sci 165:63–74. https://doi.org/10.1016/j.lfs.2016.09.008
Raimi-Abraham BT, Mahalingam S, Davies PJ, Edirisinghe M, Craig DQM (2015) Development and characterization of amorphous nanofiber drug dispersions prepared using pressurized gyration. Mol Pharm 12:3851–3861. https://doi.org/10.1021/acs.molpharmaceut.5b00127
Katti DS, Robinson KW, Ko FK, Laurencin CT (2004) Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res Part B Appl Biomater 70:286–296. https://doi.org/10.1002/jbm.b.30041
Xiao ZB, Guo MM, Guo RK (2014) Thermal decomposition mechanism and kinetics of ursolic acid and caffeic acid. Chem Ind For Prod 34:33–39. https://doi.org/10.3969/j.issn.0253-2417.2014.02.006
Akgün M, Başaran I, Suner SC, Oral A (2019) Geraniol and cinnamaldehyde as natural antibacterial additives for poly(lactic acid) and their plasticizing effects. J Polym Eng. https://doi.org/10.1515/polyeng-2019-0198
Maiza M, Benaniba MT, Quintard G, Massardier-Nageotte V (2015) Biobased additive plasticizing polylactic acid (PLA). Polimeros 25:581–590. https://doi.org/10.1590/0104-1428.1986
Gao N, Guo M, Fu Q, He Z (2017) Application of hot melt extrusion to enhance the dissolution and oral bioavailability of oleanolic acid. Asian J Pharm Sci 12:66–72. https://doi.org/10.1016/j.ajps.2016.06.006
Blasi P, Schoubben A, Giovagnoli S, Perioli L, Ricci M, Rossi C (2007) Ketoprofen poly(lactide-co-glycolide) physical interaction. AAPS PharmSciTech 8:1–8. https://doi.org/10.1208/pt0802037
Shawe J, Riesen R, Widmann J, Schubnell M (2000) UserCom. Mettler TOLEDO. 1 28
Wegiel LA, Mauer LJ, Edgar KJ, Taylor LS (2013) Crystallization of amorphous solid dispersions of resveratrol during preparation and storage-Impact of different polymers. J Pharm Sci 102:171–184. https://doi.org/10.1002/jps.23358
Irene B, Ambrogi V, Laura A, Cosimo C (2014) A hyperbranched polyester as antinucleating agent for artemisinin in electrospun nanofibers. Eur Polym J 60:145–152. https://doi.org/10.1016/j.eurpolymj.2014.09.005
Ghosh S, Kar N, Bera T (2016) Oleanolic acid loaded poly lactic co- glycolic acid- vitamin E TPGS nanoparticles for the treatment of leishmania donovani infected visceral leishmaniasis. Int J Biol Macromol 93:961–970. https://doi.org/10.1016/j.ijbiomac.2016.09.014
Basu S, Mukherjee B, Chowdhury SR, Paul P, Choudhury R, Kumar A, Mondal L, Hossain CM, Maji R (2012) Colloidal gold-loaded, biodegradable, polymer-based stavudine nanoparticle uptake by macrophages: an in vitro study. Int J Nanomedi 7:6049–6061. https://doi.org/10.2147/IJN.S38013
Teixeira ACT, Garcia AR, Ilharco LM, Gonçalves Da Silva AMPS, Fernandes AC (2010) Phase behaviour of oleanolic acid, pure and mixed with stearic acid: interactions and crystallinity. Chem Phys Lipids 163:655–666. https://doi.org/10.1016/j.chemphyslip.2010.06.001
Barbosa R, Villarreal A, Rodriguez C, De Leon H, Gilkerson R, Lozano K (2021) Aloe vera extract-based composite nanofibers for wound dressing applications. Mater Sci Eng C 124:112061. https://doi.org/10.1016/j.msec.2021.112061
Venkatesh DN, Baskaran M, Karri VVSR, Mannemala SS, Radhakrishna K, Goti S (2015) Fabrication and in vivo evaluation of nelfinavir loaded PLGA nanoparticles for enhancing oral bioavailability and therapeutic effect. Saudi Pharm J 23:667–674. https://doi.org/10.1016/j.jsps.2015.02.021
Liu Q, Ouyang WC, Zhou XH, Jin T, Wu ZW (2021) Antibacterial activity and drug loading of moxifloxacin-loaded poly(vinyl alcohol)/chitosan electrospun nanofibers. Front Mater 8:1–9. https://doi.org/10.3389/fmats.2021.643428
Böncü TE, Ozdemir N (2022) Effects of drug concentration and PLGA addition on the properties of electrospun ampicillin trihydrate-loaded PLA nanofibers. Beilstein J Nanotechnol 13:245–254. https://doi.org/10.3762/bjnano.13.19
Jyothi NVN, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, Srawan GY (2010) Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul 27:187–197. https://doi.org/10.3109/02652040903131301
Barichello JM, Morishita M, Takayama K, Nagai T (1999) Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm 25:471–476. https://doi.org/10.1081/DDC-100102197
Rodriguez C, Padilla V, Lozano K, Ahmad F, Chapa A, Villarreal A, McDonald A, Materon L, Gilkerson R (2022) Cell proliferative properties of Forcespinning® nopal composite nanofibers. J Bioact Compat Polym 37:28–37. https://doi.org/10.1177/08839115211060404
Acknowledgements
The authors acknowledge Mr. Bryan Hoke for his technical support in PXRD analysis. The authors acknowledge Ms. Casandra Lira for her support during fiber fabrication. Authors gratefully acknowledge the support received from National Science Foundation under PREM award DMR 2122178.
Author information
Authors and Affiliations
Contributions
SA, AN and KL conceived the project. SA carried out the experiments. SA conducted data analysis and drafted the manuscript. RG carried out the cell viability and cell interaction experiments and drafted the biological section of the manuscript. VMP provided technical support on SEM. VMP and KL edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests or personal/financial contributed conflicts that could influence the work imparted in this manuscript.
Additional information
Handling Editor: Jaime Grunlan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, S., Padilla-Gainza, V.M., Gilkerson, R. et al. Processing-structure–property relationships of oleanolic acid loaded PLGA fiber membranes. J Mater Sci 58, 4240–4255 (2023). https://doi.org/10.1007/s10853-023-08246-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08246-4