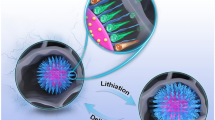
Abstract
Highly defective carbon nanotubes (HD-CNTs) are fabricated by template carbonization of polydopamine nanofilm coated on mesoporous titanium nitride nanotube arrays. With introduction of mesoporous holes on the side walls from the template, HD-CNTs exhibit much improved electrochemical properties. An ideal Nernst behavior toward [Fe(CN)6]3−/4− redox couple was observed on the HD-CNTs, with a heterogeneous charge transfer rate constant k0 greater than 0.5 cm/s that is among the fastest kinetics of electrode materials. Sensitive determination of dopamine has been demonstrated in the presence of ascorbic acid and uric acid, with a linear range of 0.1–180 μM, a detection limit of 0.074 μM (S/N = 3) and a sensitivity of 555 µA mM−1 cm−2. The excellent electrochemical activity should be attributed to the large number of exposed edge planes on the carbon nanofilm, the free-standing highly conductive titanium nitride architecture that is beneficial for electron transfer, mesoporous morphology that promotes the mass transfer between the nanotubes, and effective ion-accessible surface area.
Graphical Abstract









Similar content being viewed by others
References
Wang ZX, Zhao HL, Chen KC, Zhou FF, Magdassi S, Lan MB (2022) Two-dimensional mesoporous nitrogen-rich carbon nanosheets loaded with CeO2 nanoclusters as nanozymes for the electrochemical detection of superoxide anions in HepG2 cells. Biosens Bioelectron 209:114229. https://doi.org/10.1016/j.bios.2022.114229
Cho IH, Kim DH, Park S (2020) Electrochemical biosensors: perspective on functional nanomaterials for on-site analysis. Biomater Res 24:1–6. https://doi.org/10.1186/s40824-019-0181-y
Maduraiveeran G, Kundu M, Sasidharan M (2018) Electrochemical detection of hydrogen peroxide based on silver nanoparticles via amplified electron transfer process. J Mater Sci 53:8328–8338. https://doi.org/10.1007/s10853-018-2141-7
Zhai WZ, Srikanth N, Kong LB, Zhou K (2017) Carbon nanomaterials in tribology. Carbon 119:150–171. https://doi.org/10.1016/j.carbon.2017.04.027
Ni JF, Li Y (2016) Carbon nanomaterials in different dimensions for electrochemical energy storage. Adv Energy Mater 6:1600278. https://doi.org/10.1002/aenm.201600278
Cardenas-Benitez B, Djordjevic I, Hosseini S, Madou MJ, Martinez-Chapa SO (2018) Review-covalent functionalization of carbon nanomaterials for biosensor applications: an update. J Electrochem Soc 165:B103. https://doi.org/10.1149/2.0381803jes
Dai LM, Chang DW, Baek JB, Lu W (2012) Carbon nanomaterials for advanced energy conversion and storage. Small 8:1130–1166. https://doi.org/10.1002/smll.201101594
Yu J, Zhang YY, Li H, Wan QJ, Li YW, Yang NJ (2018) Electrochemical properties and sensing applications of nanocarbons: a comparative study. Carbon 129:301–309. https://doi.org/10.1016/j.carbon.2017.11.092
Chen GH, Dodson B, Hedges DM, Steffensen SC, Harb JN, Puleo C, Galligan C, Ashe J, Vanfleet R, Davis R (2018) Fabrication of high aspect ratio millimeter-tall free-standing carbon nanotube-based microelectrode arrays. ACS Biomater Sci Eng 4:1900–1907. https://doi.org/10.1021/acsbiomaterials.8b00038
Bulusheva LG, Arkhipov VE, Fedorovskaya EO, Zhang S, Kurenya AG, Kanygin MA, Asanov IP, Tsygankova AR, Chen XH, Song HH, Okotrub AV (2016) Fabrication of free-standing aligned multiwalled carbon nanotube array for Li-ion batteries. J Power Sources 311:42–48. https://doi.org/10.1016/j.jpowsour.2016.02.036
Liu YL, Wan LL, Wang J, Cheng L, Chen RS, Ni HW (2020) Binary electrocatalyst composed of Mo2C nanocrystals with ultra-low Pt loadings anchored in TiO2 nanotube arrays for hydrogen evolution reaction. Appl Surf Sci 509:144679. https://doi.org/10.1016/j.apsusc.2019.144679
Mohammadniaei M, Park C, Min JH, Sohn H, Lee T (2018) Fabrication of electrochemical -based bioelectronic device and biosensor composed of biomaterial-nanomaterial hybrid. Biomim Med Mater 1064:263–296. https://doi.org/10.1007/978-981-13-0445-3_17
Schroeder V, Savagatrup S, He M, Lin SB, Swager TM (2018) Carbon nanotube chemical sensors. Chem Rev 119:599–663. https://doi.org/10.1021/acs.chemrev.8b00340
Barsan MM, Ghica ME, Brett CMA (2015) Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: a review. Anal Chim Acta 881:1–23. https://doi.org/10.1016/j.aca.2015.02.059
Liu XQ, Yan R, Zhang JM, Zhu J, Wong DKY (2015) Evaluation of a carbon nanotube -titanate nanotube nanocomposite as an electrochemical biosensor scaffold. Biosens Bioelectron 66:208–215. https://doi.org/10.1016/j.bios.2014.11.028
Wang J, Liu YL, Cheng L, Chen RS, Ni HW (2020) Quasi-aligned nanorod arrays composed of nickel-cobalt nanoparticles anchored on TiO2/C nanofiber arrays as free standing electrode for enzymeless glucose sensors. J Alloy Compd 821:153510. https://doi.org/10.1016/j.jallcom.2019.153510
Briza PL, Arben M (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16. https://doi.org/10.1007/s00604-012-0871-9
Farzin L, Shamsipur M, Samandari L, Sheibani S (2018) Advances in the design of nanomaterial-based electrochemical affinity and enzymatic biosensors for metabolic biomarkers: a review. Microchim Acta 185:276. https://doi.org/10.1007/s00604-018-2820-8
McCreery RL (2008) Advanced carbon electrode materials for molecular electrochemistry. Chem Rev 108:2646–2687. https://doi.org/10.1021/cr068076m
Goncalves LM, McAuley CB, Barros AA, Compton RG (2010) Electrochemical oxidation of adenine: a mixed adsorption and diffusion response on an edge-plane pyrolytic graphite electrode. J Phys Chem C 114:14213–14219. https://doi.org/10.1021/jp1046672
Zhao JY, Cheng L, Wang J, Liu YL, Yang J, Xu QZ, Chen RS, Ni HW (2019) Heteroatom -doped carbon nanofilm embedded in highly ordered TiO2 nanotube arrays by thermal nitriding with enhanced electrochemical activity. J Electroanal Chem 852:113513. https://doi.org/10.1016/j.jelechem.2019.113513
Chen RS, Li YY, Huo KF, Chu PK (2013) Microelectrode arrays based on carbon nanomaterials: emerging electrochemical sensors for biological and environmental applications. RSC Adv 3:18698–18715. https://doi.org/10.1039/c3ra43033b
Komori K, Tatsuma T, Sakai Y (2016) Direct electron transfer kinetics of peroxidase at edge plane sites of cup-stacked carbon nanofibers and their comparison with single-walled carbon nanotubes. Langmuir 32:9163–9170. https://doi.org/10.1021/acs.langmuir.6b02407
Pan FP, Li BY, Sarnello E, Fei YH, Gang Y, Xiang XM, Du ZC, Zhang P, Wang GF, Nguyen HT, Li T, Hu YH, Zhou HC, Li Y (2020) Atomically dispersed iron-nitrogen sites on hierarchically mesoporous carbon nanotube and graphene nanoribbon networks for CO2 reduction. ACS Nano 14:5506–5516. https://doi.org/10.1021/acsnano.9b09658
Lei R, Ni HW, Chen RS, Gu HZ, Zhang H, Dong S (2018) In situ growth of self-supported and defect-engineered carbon nanotube networks on 316L stainless steel as binder-free supercapacitors. J Colloid Interface Sci 532:622–629. https://doi.org/10.1016/j.jcis.2018.08.035
Mohan R, Modak A, Schechter A (2019) A comparative study of plasma-treated oxygen -doped single-walled and multiwalled carbon nanotubes as electrocatalyst for efficient oxygen reduction reaction. ACS Sustain Chem Eng 7:11396–11406. https://doi.org/10.1021/acssuschemeng.9b01125
Adusei PK, Gbordzoe S, Kanakaraj SN, Hsieh YY, Alvarez NT, Fang YB, Johnson K, McConnell C, Shanov V (2020) Fabrication and study of supercapacitor electrodes based on oxygen plasma functionalized carbon nanotube fibers. J Energy Chem 40:120–131. https://doi.org/10.1016/j.jechem.2019.03.005
Hu LS, Fong CC, Zhang XM, Chan LL, Lam PK-S, Chu PK, Wong K-Y, Yang MS (2016) Au nanoparticles decorated TiO2 nanotube arrays as a recyclable sensor for photoenhanced electrochemical detection of bisphenol A. Environ Sci Technol 50:4430–4438. https://doi.org/10.1021/acs.est.5b05857
Wang Y-W, Liu Y-L, Xu JQ, Qin Y, Huang W-H (2018) Stretchable and photocatalytically renewable electrochemical sensor based on sandwich nanonetworks for real-time monitoring of cells. Anal Chem 90:5977–5981. https://doi.org/10.1021/acs.analchem.8b01396
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430. https://doi.org/10.1126/science.1147241
Wang J, Zeng Y, Wan LL, Zhao JY, Yang J, Hu J, Liang F (2020) Catalyst-free fabrication of one-dimensional N-doped carbon coated TiO2 nanotube arrays by template carbonization of polydopamine for high performance electrochemical sensors. Appl Surf Sci 509:145301. https://doi.org/10.1016/j.apsusc.2020.145301
Scroccarello A, Della Pelle F, Fratini E, Ferraro G, Scarano S, Palladino P, Compagnone D (2020) Colorimetric determination of polyphenols via a gold nanoseeds-decorated polydopamine film. Microchim Acta 187:267. https://doi.org/10.1007/s00604-020-04228-4
Yang J, Cheng L, Wan LL, Yan JB, Chen RS, Ni HW (2018) Fabrication of sandwich structured C/NiO/TiO2 nanotube arrays for enhanced electrocatalytic activity towards hydrogen evolution. Electrochem Commun 97:68–72. https://doi.org/10.1016/j.elecom.2018.10.018
Hu LB, Hecht DS, Gruner G (2010) Carbon nanotube thin films: fabrication, properties, and applications. Chem Rev 110:5790–5844. https://doi.org/10.1021/cr9002962
Chen M, Zhao G, Shao L-L, Yuan Z-Y, Jing Q-S, Huang K-J, Huang X-H, Zou G-D (2017) Controlled synthesis of nickel encapsulated into nitrogen-doped carbon nanotubes with covalent bonded interfaces: the structural and electronic modulation strategy for an efficient electrocatalyst in dye-sensitized solar cells. Chem Mater 29:9680–9694. https://doi.org/10.1021/acs.chemmater.7b03385
Lu XH, Liu TY, Zhai T, Wang GM, Yu MH, Xie SL, Ling YC, Liang CL, Tong YX, Li Y (2014) Improving the cycling stability of metal-nitride supercapacitor electrodes with a thin carbon shell. Adv Energy Mater 4:1300994. https://doi.org/10.1002/aenm.201300994
Fan HW, Zhang SY, Zhu XF (2019) Nitrided TiO2 nanoparticles/nanotube arrays for better electrochemical properties. Chem Phys Lett 730:340–344. https://doi.org/10.1016/j.cplett.2019.06.025
Sun P, Lin R, Wang ZL, Qiu MJ, Chai ZS, Zhang BD, Meng H, Tan SZ, Zhao CX, Mai WJ (2017) Rational design of carbon shell endows TiN@C nanotube based fiber supercapacitors with significantly enhanced mechanical stability and electrochemical performance. Nano Energy 31:432–440. https://doi.org/10.1016/j.nanoen.2016.11.052
Cheng L, Li YY, Liu YL, Wang J, Chen RS, Ni HW (2019) One-dimensional nitrogen-doped carbon nanotube arrays fabricated by template carbonization over anodized TiO2 nanotube arrays. Mater Lett 256:126602. https://doi.org/10.1016/j.matlet.2019.126602
Zhao JY, Zeng Y, Wang J, Xu QZ, Chen RS, Ni HW, Cheng GJ (2020) Ultrahigh electrocatalytic activity with trace amounts of platinum loadings on free-standing mesoporous titanium nitride nanotube arrays for hydrogen evolution reactions. Nanoscale 12:15393–15401. https://doi.org/10.1039/d0nr01316a
Wang JL, Meng Z, Yan XF, Ying HJ, Zhang SL, Han WQ (2019) Facile preparation of porous TiN-C microspheres as an efficient sulfur host for high performance lithium-sulfur battery. Mater Today Energy 13:1–10. https://doi.org/10.1016/j.mtener.2019.04.010
Chen RS, Hu LS, Huo KF, Fu JJ, Ni HW, Tang Y, Chu PK (2011) Controllable growth of conical and cylindrical TiO2-carbon core-shell nanofiber arrays and morphologically dependent electrochemical properties. Chem Eur J 17:14552–14558. https://doi.org/10.1002/chem.201102219
Mao ZY, Chen JJ, Yang YF, Bie LJ, Fahlman BD, Wang DJ (2017) Modification of surface properties and enhancement of photocatalytic performance for g-C3N4 via plasma treatment. Carbon 123:651–659. https://doi.org/10.1016/j.carbon.2017.08.020
Ensafi AA, Arashpour B, Rezaei B, Allafchian AR (2014) Voltammetric behavior of dopamine at a glassy carbon electrode modified with NiFe2O4 magnetic nanoparticles decorated with multiwall carbon nanotubes. Mater Sci Eng C Mater Biol Appl 39:78. https://doi.org/10.1016/j.msec.2014.02.024
Bali Prasad B, Jauhari D, Tiwari MP (2013) A dual-template imprinted polymer-modified carbon ceramic electrode for ultra trace simultaneous analysis of ascorbic acid and dopamine. Biosens Bioelectron 50:19. https://doi.org/10.1016/j.bios.2013.05.062
Rezaei B, Boroujeni MK, Ensafi AA (2015) Fabrication of DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer electrochemical sensor for the determination of dopamine. Biosens Bioelectron 66:490. https://doi.org/10.1016/j.bios.2014.12.009
Zhu W, Chen T, Ma X, Ma H, Chen S (2013) Highly sensitive and selective detection of dopamine based on hollow gold nanoparticles-graphene nanocomposite modified electrode. Colloid Surf B 111:321. https://doi.org/10.1016/j.colsurfb.2013.06.026
Rezaei B, Havakeshian E, Ensafi AA (2015) Decoration of nanoporous stainless steel with nanostructured gold via galvanic replacement reaction and its application for electrochemical determination of dopamine. Sens Actuat B-Chem 213:484. https://doi.org/10.1016/j.snb.2015.02.106
Wu D, Li Y, Zhang Y, Wang P, Wei Q, Du B (2014) Sensitive electrochemical sensor for simultaneous determination of dopamine, ascorbic acid, and uric acid enhanced by amino-group functionalized mesoporous Fe3O4@graphene sheets. Electrochim Acta 116:244. https://doi.org/10.1016/j.electacta.2013.11.033
Chiniforoshan H, Ensafi AA, Heydari-Bafrooei E, Khalesi SB, Tabrizi L (2015) Polymeric nanoparticle of copper(II)-4,4′-dicyanamidobiphenyl ligand: synthetic, spectral and structural aspect; application to electrochemical sensing of dopamine and ascorbic acid. Appl Surf Sci 347:315. https://doi.org/10.1016/j.apsusc.2015.04.062
Sun X, Zhang L, Zhang X, Liu X, Jian J, Kong D, Zeng D, Yuan H, Feng S (2020) Electrochemical dopamine sensor based on superionic conducting potassium ferrite. Biosens Bioelectron 153:112045. https://doi.org/10.1016/j.bios.2020.112045
Umapathi S, Masud J, Coleman H, Nath M (2020) Electrochemical sensor based on CuSe for determination of dopamine. Microchim Acta 187:440. https://doi.org/10.1007/s00604-020-04405-5
Wang S, Guo P, Ma G, Wei J, Wang Z, Cui L, Sun L, Wang A (2020) Three-dimensional hierarchical mesoporous carbon for regenerative electrochemical dopamine sensor. Electrochim Acta 360:137016. https://doi.org/10.1016/j.electacta.2020.137016
Acknowledgements
This work was supported by National Key R&D Program of China (2020YFC1909702) and National Natural Science Foundation of China (U21A20317).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Maude Jimenez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, M., Li, Y., Nie, Y. et al. Highly defective carbon nanotubes with improved electrochemical properties by introduction of mesoporous holes on the side walls. J Mater Sci 57, 20567–20579 (2022). https://doi.org/10.1007/s10853-022-07949-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07949-4