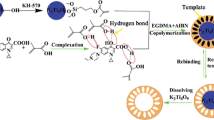
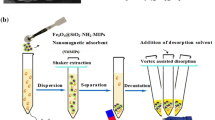
Abstract
A novel hollow porous molecularly imprinted polymer (HMIP) was synthesized for tetracycline determination in different matrices and the adsorption efficiency of this material was compared with the widely applied traditional (unmodified) and core–shell MIP structures. For this purpose, three different tetracycline imprinted polymers were obtained: with the reagent conditions optimized from traditional MIP, a core–shell MIP was obtained by surface polymerization of SiO2 nanoparticles (SiO2MIP). Subsequently, the silica core was removed, obtaining the hollow porous MIP. All materials were characterized by different techniques and HMIP presented higher surface area (227 m2 g−1) and pore volume (0.2 cm3 g−1) when compared with MIP (82.3 m2 g−1 and 0.09 cm3 g−1) or SiO2@MIP (109 m2 g−1 and 0.1 cm3 g−1) and the respective Non-Imprinted Polymers (NIPs). After optimizing the analysis conditions each polymer structure exhibited different kinetics and equilibrium adsorption behavior, which was related to greater or lesser porosity. HMIP presented superior results for the identification of tetracycline in adsorption experiments (maximum binding capacity of 5.6 mg g−1 and 0.2 L mg−1 affinity constant) and in selectivity tests. Water, synthetic urine and milk samples fortified with tetracycline were evaluated and HMIP showed recovery in the range of 74–96%.
Graphical abstract






Similar content being viewed by others
References
Gogoi A, Mazumder P, Tyagi VK, Tushara Chaminda GG, An AK, Kumar M (2018) Occurrence and fate of emerging contaminants in water environment: a review. Groundw Sustain Dev 6:169–180. https://doi.org/10.1016/j.gsd.2017.12.009
Chaturvedi P, Shukla P, Giri BS, Chowdhary P, Chandra R, Gupta P, Pandey A (2021) Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ Res 194:110664. https://doi.org/10.1016/j.envres.2020.110664
Pereira-Maia EC, Silva PP, de Almeida WB, dos Santos HF, Marcial BL, Ruggiero R, Guerra W (2010) Tetraciclinas e glicilciclinas: uma visão geral. Quim Nova 33:700–706. https://doi.org/10.1590/s0100-40422010000300038
Pérez-Rodríguez M, Pellerano RG, Pezza L, Pezza HR (2018) An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 182:1–21. https://doi.org/10.1016/j.talanta.2018.01.058
Ebele AJ, Abou-Elwafa Abdallah M, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3:1–16. https://doi.org/10.1016/j.emcon.2016.12.004
Borghi AA, Palma MSA (2014) Tetracycline: production, waste treatment and environmental impact assessment. Brazilian J Pharm Sci 50:25–40. https://doi.org/10.1590/S1984-82502011000100003
Liu X, Huang D, Lai C, Zeng G, Qin L, Zhang C, Yi H, Li B, Deng R, Liu S, Zhang Y (2018) Recent advances in sensors for tetracycline antibiotics and their applications. TrAC Trends Anal Chem 109:260–274. https://doi.org/10.1016/j.trac.2018.10.011
Pang YH, Lv ZY, Sun JC, Yang C, Shen XF (2021) Collaborative compounding of metal–organic frameworks for dispersive solid-phase extraction HPLC–MS/MS determination of tetracyclines in honey. Food Chem 355:129411. https://doi.org/10.1016/j.foodchem.2021.129411
Jia L, Chen R, Xu J, Zhang L, Chen X, Bi N, Gou J, Zhao T (2021) A stick-like intelligent multicolor nano-sensor for the detection of tetracycline: the integration of nano-clay and carbon dots. J Hazard Mater 413:125296. https://doi.org/10.1016/j.jhazmat.2021.125296
Zeb S, Wong A, Khan S, Hussain S, Sotomayor MDPT (2021) Using magnetic nanoparticles/MIP-based electrochemical sensor for quantification of tetracycline in milk samples. J Electroanal Chem 900:115713. https://doi.org/10.1016/j.jelechem.2021.115713
Bu T, Jia P, Sun X, Liu Y, Wang Q, Wang L (2020) Hierarchical molybdenum disulfide nanosheets based lateral flow immunoassay for highly sensitive detection of tetracycline in food samples. Sens Actuators B Chem 320:128440. https://doi.org/10.1016/j.snb.2020.128440
Scaria J, Anupama KV, Nidheesh PV (2021) Tetracyclines in the environment: an overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci Total Environ 771:145291. https://doi.org/10.1016/j.scitotenv.2021.145291
Minale M, Gu Z, Guadie A, Kabtamu DM, Li Y, Wang X (2020) Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: a review. J Environ Manage 276:111310. https://doi.org/10.1016/j.jenvman.2020.111310
Saghir S, Xiao Z (2021) Facile preparation of metal-organic frameworks-8 (ZIF-8) and its simultaneous adsorption of tetracycline (TC) and minocycline (MC) from aqueous solutions. Mater Res Bull 141:111372. https://doi.org/10.1016/j.materresbull.2021.111372
Luo Q, Ren T, Lei Z, Huang Y, Huang Y, Xu D, Wan C, Guo X, Wu Y (2022) Non-toxic chitosan-based hydrogel with strong adsorption and sensitive detection abilities for tetracycline. Chem Eng J 427:131738. https://doi.org/10.1016/j.cej.2021.131738
Chen W, Zhao B, Guo Y, Guo Y, Zheng Z, Pak T, Li G (2021) Effect of hydrothermal pretreatment on pyrolyzed sludge biochars for tetracycline adsorption. J Environ Chem Eng 9:106557. https://doi.org/10.1016/j.jece.2021.106557
Belbruno JJ (2018) Molecularly Imprinted Polymers. Chem Rev 119:94–119. https://doi.org/10.1021/acs.chemrev.8b00171
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942. https://doi.org/10.1039/c0cs00084a
Halhalli MR, Schillinger E, Aureliano CSA, Sellergren B (2012) Thin walled imprinted polymer beads featuring both uniform and accessible binding sites. Chem Mater 24:2909–2919. https://doi.org/10.1021/cm300965t
Wackerlig J, Schirhagl R (2016) Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: a review. Anal Chem 88:250–261. https://doi.org/10.1021/acs.analchem.5b03804
Madhuri R, Roy E, Gupta K, Sharma PK (2014) Combination of Molecular Imprinting and Nanotechnology: Beginning of a New Horizon. Adv Biomater Biodevices 367–422. https://doi.org/10.1002/9781118774052.ch11
Niu M, Pham-Huy C, He H (2016) Core-shell nanoparticles coated with molecularly imprinted polymers: a review. Microchim Acta 183:2677–2695. https://doi.org/10.1007/s00604-016-1930-4
Fan D, Li H, Shi S, Chen X (2016) Hollow molecular imprinted polymers towards rapid, effective and selective extraction of caffeic acid from fruits. J Chromatogr A 1470:27–32. https://doi.org/10.1016/j.chroma.2016.10.006
Prasad BB, Singh K (2017) Molecularly imprinted polymer-based core-shells (solid vs hollow) @ pencil graphite electrode for electrochemical sensing of certain anti-HIV drugs. Sens Actuators B Chem 244:167–174. https://doi.org/10.1016/j.snb.2016.12.109
Bhogal S, Kaur K, Malik AK, Sonne C, Lee SS, Kim KH (2020) Core-shell structured molecularly imprinted materials for sensing applications. TrAC Trends Anal Chem 133:116043. https://doi.org/10.1016/j.trac.2020.116043
Xu S, Chen L, Li J, Qin W, Ma J (2011) Preparation of hollow porous molecularly imprinted polymers and their applications to solid-phase extraction of triazines in soil samples. J Mater Chem 21:12047–12053. https://doi.org/10.1039/c1jm10905g
Pizan-Aquino C, Wong A, Avilés-Félix L, Khan S, Picasso G, Sotomayor MDPT (2020) Evaluation of the performance of selective M-MIP to tetracycline using electrochemical and HPLC-UV method. Mater Chem Phys 245. https://doi.org/10.1016/j.matchemphys.2020.122777
Hu X, Zhao Y, Dong J, Liu C, Qi Y, Fang G, Wang S (2021) A strong blue fluorescent nanoprobe based on Mg/N co-doped carbon dots coupled with molecularly imprinted polymer for ultrasensitive and highly selective detection of tetracycline in animal-derived foods. Sens Actuators B Chem 338:129809. https://doi.org/10.1016/j.snb.2021.129809
Denobile M, De Souza NE (2004) Validação de método para determinação de resíduos oxitetraciclina, tetraciclina, clortetaciclina e doxiciclina, em leite, por cromatografia líquida de alta eficiência. Rev Bras Ciencias Farm J Pharm Sci 40:209–218
Shi S, Fan D, Xiang H, Li H (2017) Effective synthesis of magnetic porous molecularly imprinted polymers for efficient and selective extraction of cinnamic acid from apple juices. Food Chem 237:198–204. https://doi.org/10.1016/j.foodchem.2017.05.086
Silva CR, Jardim ICSF, Collins CH, Airoldi C (2004) Novas fases estacionárias à base de sílica para cromatografia líquida de alta eficiência. Quim Nova 27:270–276. https://doi.org/10.1590/S0100-40422004000200017
Snyder LR, Kirkland JJ, Dolan JW (2010) The column. Introduction to modern liquid chromatography, 3rd edn. Wiley, New Jersey, pp 199–252
Zhang Z, Chen L, Yang F, Li J (2014) Uniform core-shell molecularly imprinted polymers: a correlation study between shell thickness and binding capacity. RSC Adv 4:31507–31514. https://doi.org/10.1039/c4ra03282a
Xiang H, Fan D, Li H, Shi S (2017) Hollow porous molecularly imprinted polymers for rapid and selective extraction of cinnamic acid from juices. J Chromatogr B Anal Technol Biomed Life Sci 1049–1050:1–7. https://doi.org/10.1016/j.jchromb.2017.02.014
Hu X, Xie L, Guo J, Li H, Jiang X, Zhang Y, Shi S (2015) Hydrophilic gallic acid–imprinted polymers over magnetic mesoporous silica microspheres with excellent molecular recognition ability in aqueous fruit juices. Food Chem 179:206–212. https://doi.org/10.1016/j.foodchem.2015.02.007
Xie L, Guo J, Zhang Y, Hu Y, You Q, Shi S (2015) Novel molecular imprinted polymers over magnetic mesoporous silica microspheres for selective and efficient determination of protocatechuic acid in Syzygium aromaticum. Food Chem 178:18–25. https://doi.org/10.1016/j.foodchem.2015.01.069
Funding
The authors would like to thank the National Institute for Alternative Technologies of Detection, Toxicological Evaluation and Removal of Micropollutants and Radioactives (INCT-DATREM), National Council for Scientific and Technological Development—CNPq—(465571/2014-0) and São Paulo Research Foundation—FAPESP—(Grant Numbers #2014/50945-4, #2017/20789-9 and #2019/00677-7) for the financial support in the development of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Additional information
Handling Editor: Gregory Rutledge.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pupin, R.R., Sotomayor, M.D.P.T. Synthesis and evaluation of hollow porous molecularly imprinted polymer for selective determination of tetracycline. J Mater Sci 57, 17291–17303 (2022). https://doi.org/10.1007/s10853-022-07727-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07727-2