Abstract
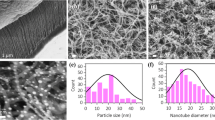
We prepared metal nano-particles deposited on SWCNTs by heat-treatment of SWCNTs encapsulating metal complex (Ni(acac)2, Cu(acac)2) molecules inside their hollow cores. It was confirmed by synchrotron radiation x-ray absorption spectra that the obtained nano-particles were metallic Ni and Cu. Electron microscopic measurements revealed that the diameters of most of the metal particles are less than 10 nm and that they were well dispersed on SWCNTs even in the bundles. Ni metal particles on SWCNTs were converted to Ni(OH)2 particles by electrochemical treatment in 1 M KOH aqueous solution. We investigated the Ni-MH battery electrode properties of the obtained Ni(OH)2/SWCNT sample. We expect Ni(OH)2/SWCNT to work as high-power battery electrode, because the reversible charge/discharge capacity of Ni(OH)2/SWCNT did not change much with increasing the scan rate from 2 to 1000 mV/s. Cu/SWCNT worked as a CO2 photo-reduction catalyst by combing it with light-absorbing semiconductors. In the photo-catalyst, Cu works as a metal co-catalyst to enhance the reaction efficiency. We used inorganic semiconductor TiO2 and organic semiconductor epindolidine (EPI). It was found that both Cu/SWCNT/TiO2 and Cu/SWCNT/EPI can work as CO2 photo-reduction catalyst. The external CO2 to CO quantum yield of Cu/SWCNT/EPI catalyst under the simulated solar light (AM1.5G, 100 mW/cm2) was 2.2 × 10−4%.
Graphical abstract








Similar content being viewed by others
References
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58. https://doi.org/10.1038/354056a0
Iijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature 363:603–605. https://doi.org/10.1038/363603a0
Bethune DS, Kiang CH, de Vries MS, Gorman G, Savoy R, Vazquez J, Beyers R (1993) Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 363:605–607. https://doi.org/10.1038/363605a0
Saito Y, Uemura S, Hamaguchi K (1998) Cathode ray tube lighting elements with carbon nanotube field emitters. Jpn J Appl Phys 37:L346–L348. https://doi.org/10.1143/jjap.37.l346
Mizutani T, Iwatsuki S, Ohno Y, Kishimoto S (2005) Effects of fabrication process on current-voltage characteristics of carbon nanotube field effect transistors. Jpn J Appl Phys 44:1599–1602. https://doi.org/10.1143/jjap.44.1599
Ajayan PM, Schadler LS, Giannaris C, Rubio A (2000) Single-walled carbon nanotube-polymer composites: strength and weakness. Adv Mater 12:750–753. https://doi.org/10.1002/(SICI)1521-4095(200005)12:10%3c750::AID-ADMA750%3e3.0.CO;2-6
Zhou O, Fleming RM, Murphy DW, Chen CH, Haddon RC, Ramirez AP, Glarum SH (1994) Defects in carbon nanostructures. Science 263:1744–1747. https://doi.org/10.1126/science.263.5154.1744
Zhao J, Buldum A, Han J, Lu JP (2000) First-principles study of Li-intercalated carbon nanotube ropes. Phys Rev Lett 85:1706–1709
Shiraishi S, Kurihara H, Okabe K, Hulicova D, Oya A (2002) Electric double layer capacitance of highly pure single-walled carbon nanotubes (HiPco™Buckytubes™) in propylene carbonate electrolytes. Electrochem Commun 4:593–598. https://doi.org/10.1016/S1388-2481(02)00382-X
Nomura A, Ito K, Kubo Y (2017) CNT sheet air electrode for the development of ultra-high cell capacity in lithium-air batteries. Sci Rep 7:45596. https://doi.org/10.1038/srep45596
Ikonen T, Kalidas N, Lahtinen K, Isoniemi T, Toppari JJ, Vázquez E, Herrero-Chamorro MA, Fierro JLG, Kallio T, Lehto V-P (2020) Conjugation with carbon nanotubes improves the performance of mesoporous silicon as Li-ion battery anode. Sci Rep. https://doi.org/10.1038/s41598-020-62564-0
Coelho J, Pokle A, Park S-H, McEvoy N, Berner NC, Duesberg GS, Nicolosi V (2017) Lithium titanate/carbon nanotubes composites processed by ultrasound irradiation as anodes for lithium ion batteries. Sci Rep. https://doi.org/10.1038/s41598-017-06908-3
Smith BW, Monthioux M, Luzzi DE (1998) Encapsulated C60 in carbon nanotubes. Nature 396:323–324. https://doi.org/10.1038/24521
Hirahara K, Bandow S, Suenaga K, Kato H, Okazaki T, Shinohara H, Iijima S (2001) Electron diffraction study of one-dimensional crystals of fullerenes. Phys Rev B. https://doi.org/10.1103/physrevb.64.115420
Lee J, Kim H, Kahng SJ, Kim G, Son YW, Ihm J, Kato H, Wang ZW, Okazaki T, Shinohara H, Kuk Y (2002) Bandgap modulation of carbon nanotubes by encapsulated metallofullerenes. Nature 415:1005–1008. https://doi.org/10.1038/4151005a
Maniwa Y, Kataura H, Abe M, Suzuki S, Achiba Y, Kira H, Matsuda K (2002) Phase transition in confined water inside carbon nanotubes. J Phys Soc Jpn 71:2863–2866. https://doi.org/10.1143/jpsj.71.2863
Yanagi K, Miyata Y, Kataura H (2006) Highly stabilized β-carotene in carbon nanotubes. Adv Mater 18:437–441. https://doi.org/10.1002/adma.200501839
Shiraishi M, Swaraj S, Takenobu T, Iwasa Y, Ata M, Unger WES (2005) Spectroscopic characterization of single-walled carbon nanotubes carrier-doped by encapsulation of TCNQ. Phys Rev B. https://doi.org/10.1103/physrevb.71.125419
Song H, Ishii Y, Al-zubaidi A, Sakai T, Kawasaki S (2013) Temperature-dependent water solubility of iodine-doped single-walled carbon nanotubes prepared using an electrochemical method. Phys Chem Chem Phys 15:5767–5770. https://doi.org/10.1039/C3CP50506E
Chen W, Jin Y, Zhao J, Liu N, Cui Y (2018) Nickel-hydrogen batteries for large-scale energy storage. Proc Natl Acad Sci USA 115:11694–11699. https://doi.org/10.1073/pnas.1809344115
Olabi AG (2017) Renewable energy and energy storage systems. Energy 136:1–6. https://doi.org/10.1016/j.energy.2017.07.054
Amrouche SO, Rekioua D, Rekioua T, Bacha S (2016) Overview of energy storage in renewable energy systems. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2016.06.243
Al-Zubaidi A, Kobayashi K, Ishii Y, Kawasaki S (2021) One-step synthesis of visible light CO2 reduction photocatalyst from carbon nanotubes encapsulating iodine molecules. Sci Rep. https://doi.org/10.1038/s41598-021-89706-2
Zhao J, Xue S, Barber J, Zhou Y, Meng J, Ke X (2020) An overview of Cu-based heterogeneous electrocatalysts for CO2 reduction. J Mater Chem A 8:4700–4734. https://doi.org/10.1039/c9ta11778d
Devi P, Malik K, Arora E, Bhattacharya S, Kalendra V, Lakshmi KV, Verma A, Singh JP (2019) Selective electrochemical reduction of CO2 to CO on CuO/In2O3 nanocomposites: role of oxygen vacancies. Catal Sci Technol 9:5339–5349. https://doi.org/10.1039/c9cy01396b
Nagai K, Kuwabara T, Ahmad MF, Nakano M, Karakawa M, Taima T, Takahashi K (2019) High performance photoanodic catalyst prepared from an active organic photovoltaic cell—high potential gain from visible light. Chem Commun 55:12491–12494. https://doi.org/10.1039/c9cc04759j
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44:8269–8285. https://doi.org/10.1143/jjap.44.8269
Zhang Z, Long J, Yang L, Chen W, Dai W, Fu X, Wang X (2011) Organic semiconductor for artificial photosynthesis: water splitting into hydrogen by a bioinspired C3N3S3polymer under visible light irradiation. Chem Sci 2:1826–1830. https://doi.org/10.1039/c1sc00257k
Jakešová M, Apaydin DH, Sytnyk M, Oppelt K, Heiss W, Sariciftci NS, Głowacki ED (2016) Hydrogen-bonded organic semiconductors as stable photoelectrocatalysts for efficient hydrogen peroxide photosynthesis. Adv Funct Mater 26:5248–5254. https://doi.org/10.1002/adfm.201601946
Gryszel M, Sytnyk M, Jakešová M, Romanazzi G, Gabrielsson R, Heiss W, Głowacki ED (2018) General observation of photocatalytic oxygen reduction to hydrogen peroxide by organic semiconductor thin films and colloidal crystals. ACS Appl Mater Interfaces 10:13253–13257. https://doi.org/10.1021/acsami.8b01295
Głowacki ED, Romanazzi G, Yumusak C, Coskun H, Monkowius U, Voss G, Burian M, Lechner RT, Demitri N, Redhammer GJ, Sünger N, Suranna GP, Sariciftci S (2015) Epindolidiones-versatile and stable hydrogen-bonded pigments for organic field-effect transistors and light-emitting diodes. Adv Funct Mater 25:776–787. https://doi.org/10.1002/adfm.201402539
K. Kondo, Y. Watanabe, J. Kuno, Y. Ishii, S. Kawasaki, M. Kato, G. Kalita, Y. Hattori, O. Mashkov, M. Sytnyk, W. Heiss. Flexible photocatalytic electrode using graphene, non-noble metal, and organic semiconductors for hydrogen evolution reaction. Energy Technol. https://doi.org/10.1002/ente.202100123
Yoshida Y, Ishii Y, Kato N, Li C, Kawasaki S (2016) Low-temperature phase transformation accompanied with charge-transfer reaction of polyiodide ions encapsulated in single-walled carbon nanotubes. J Phys Chem C 120:20454–20461. https://doi.org/10.1021/acs.jpcc.6b07819
Kato N, Ishii Y, Yoshida Y, Sakamoto Y, Matsushita K, Takahashi M, Date R, Kawasaki S (2019) Electrochemical reactions of iodine molecules encapsulated in single-walled carbon nanotubes. ACS Omega 4:2547–2553. https://doi.org/10.1021/acsomega.8b03129
Al-Zubaidi A, Asai N, Ishii Y, Kawasaki S (2020) The effect of diameter size of single-walled carbon nanotubes on their high-temperature energy storage behaviour in ionic liquid-based electric double-layer capacitors. RSC Adv 10:41209–41216. https://doi.org/10.1039/d0ra08579k
Zhu Y, Cao C, Tao S, Chu W, Wu Z, Li Y (2015) Ultrathin nickel hydroxide and oxide nanosheets: synthesis, characterizations and excellent supercapacitor performances. Sci Rep 4:5787. https://doi.org/10.1038/srep05787
Morikawa T, Gul S, Nishimura YF, Suzuki TM, Yano J (2020) Operando X-ray absorption spectroscopy of hyperfine β-FeOOH nanorods modified with amorphous Ni(OH)2 under electrocatalytic water oxidation conditions. Chem Commun 56:5158–5161. https://doi.org/10.1039/d0cc00692k
Sharma A, Varshney M, Park J, Ha T-K, Chae K-H, Shin H-J (2015) XANES, EXAFS and photocatalytic investigations on copper oxide nanoparticles and nanocomposites. RSC Adv 5:21762–21771. https://doi.org/10.1039/c4ra16217j
Acknowledgements
This work was supported by the JSPS Japanese-German Graduate Externship (Grant No. 2019/R1), JSPS KAKENHI Grant Numbers 19H02809, 19K15502 and 20K20946, the Sumitomo Foundation, the Hibi Science Foundation, the Thermal & Electric Energy Technology Foundation, and the Society of Iodine Science. W. H. was supported by the Project GRK2495/J from the Deutsche Forschungsgemeinschaft (DFG).
Funding
Funding was provided by Jsps Japanese-German Graduate Externship (Grant Number 2019/R1), Japan Society for the Promotion of Science (Grant Numbers KAKENHI 19H02809, 19K15502, 20K20946), Sumitomo Foundation, Hibi Science Foundation, Thermal and Electric Energy Technology Foundation, Society of Iodine Science, and Deutsche Forschungsgemeinschaft (GRK2495/J).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial/commercial conflicts of interest associated with this manuscript.
Additional information
Handling Editor: Mark Bissett.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ishii, Y., Ishikawa, S., Yamada, I. et al. Ultra-fine metal particles dispersed on single-walled carbon nanotubes for energy devices. J Mater Sci 57, 4300–4310 (2022). https://doi.org/10.1007/s10853-022-06894-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-06894-6