Abstract
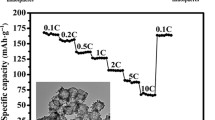
Based on hydrothermal synthesis and solid-phase thermal reaction, LiNi0.8Co0.15Al0.05O2 hollow nanospheres (LNCA HNSs) were synthesized by using SiO2 hollow nanospheres as hard template. Firstly, the SiO2 HNSs were prepared. Then, (Ni0.8Co0.15Al0.05)CO3 nanosheets grew on the surface of SiO2 HNSs to form SiO2@(Ni0.8Co0.15Al0.05)CO3 hollow nanospheres with double shells by hydrothermal method. Finally, the above precursors and lithium source were calcined at high temperature, and then SiO2 template was etched to obtain hollow LNCA HNSs. The characterization results showed that the LNCA HNSs are hollow spheres with a diameter of about 1.8 μm. The shell thickness of LNCA HNSs is about 300 nm. Compared with LNCA nanoparticles and LNCA microparticles, LNCA HNSs showed excellent stability, high capacity, and good rate performance as cathode materials for lithium ion batteries. The LNCA HNSs exhibited a reversible capacity of 202.4 mA h g−1 at 0.1 C and good stability of 179.1 mA h g−1 at 1 C after 80 cycles.







Similar content being viewed by others
References
Choi NS, Chen ZH, Freunberger SA, Ji XL, Sun YK, Amine K, Yushin G, Nazar LF, Cho J, Bruce PG (2012) Challenges facing lithium batteries and electrical double-layer capacitors. Angew Chem-Int Ed 51(40):9994–10024
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414(6861):359–367
Poizot P, Dolhem F (2011) Clean energy new deal for a sustainable world: from non-CO2 generating energy sources to greener electrochemical storage devices. Energy Environ Sci 4(6):2003–2019
Scrosati B, Hassoun J, Sun YK (2011) Lithium-ion batteries. A look into the future. Energy Environ Sci 4(9):3287–3295
Park M, Zhang XC, Chung MD, Less GB, Sastry AM (2010) A review of conduction phenomena in Li-ion batteries. J Power Sources 195(24):7904–7929
Sun HX, Du HR, Yu MK, Huang KF, Yu N, Geng BY (2019) Vesicular Li3V2(PO4)(3)/C hollow mesoporous microspheres as an efficient cathode material for lithium-ion batteries. Nano Res 12(8):1937–1942
Liu W, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed 54(15):4440–4457
Myung ST, Maglia F, Park KJ, Yoon CS, Lamp P, Kim SJ, Sun YK (2017) Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. ACS Energy Lett 2(1):196–223
Qiu L, Xiang W, Tian W, Xu CL, Li YC, Wu ZG, Chen TR, Jia K, Wang D, He FR, Guo XD (2019) Polyanion and cation co-doping stabilized Ni-rich Ni–Co–Al material as cathode with enhanced electrochemical performance for Li-ion battery. Nano Energy 63:9. https://doi.org/10.1016/j.nanoen.2019.06.014
Ryu HH, Park KJ, Yoon CS, Sun YK (2018) Capacity fading of Ni-Rich Li NixCoyMn1−x−yO−2 (0.6≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation? Chem Mater 30(3):1155–1163
Vadivel S, Phattharasupakun N, Wutthiprom J, Duangdangchote S, Sawangphruk M (2019) High-performance Li-ion batteries using nickel-rich lithium nickel cobalt aluminium oxide-nanocarbon core-shell cathode: in operando X-ray diffraction. ACS Appl Mater Interfaces 11(34):30719–30727
Chen T, Li X, Wang H, Yan XX, Wang L, Deng BW, Ge WJ, Qu MZ (2018) The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. J Power Sources 374:1–11
Mukherjee P, Faenza NV, Pereira N, Ciston J, Piper LFJ, Amatucci GG, Cosandey F (2018) Surface structural and chemical evolution of layered LiNi0.8Co0.15Al0.050O2 (NCA) under high voltage and elevated temperature conditions. Chem Mater 30(23):8431–8445
Zheng JC, Yang Z, He ZJ, Tong H, Yu WJ, Zhang JF (2018) In situ formed LiNi0.8Co0.15Al0.05O2@Li4SiO4 composite cathode material with high rate capability and long cycling stability for lithium-ion batteries. Nano Energy 53:613–621
Li J, Harlow J, Stakheiko N, Zhang N, Paulsen J, Dahn J (2018) Dependence of cell failure on cut-off voltage ranges and observation of kinetic hindrance in LiNi0.8Co0.15Al0.05O2. J Electrochem Soc 165(11):A2682–A2695
Zhu XH, Revilla RI, Jaguemont J, Van Mierlo J, Hubin A (2019) Insights into cycling aging of LiNi0.8Co0.15Al0.05O2 cathode induced by surface inhomogeneity: a post-mortem analysis. J Phys Chem C 123(50):30046–30058
Li XR, Xiao X, Li Q, Wei JL, Xue HG, Pang H (2018) Metal (M = Co, Ni) phosphate based materials for high-performance supercapacitors. Inorg Chem Front 5(1):11–28
Zhang HH, Guan B, Gu JN, Li Y, Ma C, Zhao J, Wang TY, Cheng CJ (2016) One-step synthesis of nickel cobalt sulphides particles: tuning the composition for high performance supercapacitors. RSC Adv 6(64):58916–58924
Hou PY, Zhang HZ, Deng XL, Xu XJ, Zhang LQ (2017) Stabilizing the electrode/electrolyte interface of LiNi0.8Co0.15Al0.05O2 through tailoring aluminum distribution in microspheres as long-life, high-rate, and safe cathode for lithium-ion batteries. ACS Appl Mater Interfaces 9(35):29643–29653
Kim Y, Kim D (2012) Synthesis of high-density nickel cobalt aluminum hydroxide by continuous coprecipitation method. ACS Appl Mater Interfaces 4(2):586–589
Natarajan S, Moodakare SB, Shanmugam V, Haridoss P, Gopalan R (2018) Infrared spectroscopy signatures of aluminum segregation and partial oxygen substitution by sulfur in LiNi0.8Co0.15Al0.05O2. ACS Appl Energy Mater 1(6):2536–2545
Yang XR, Chen JW, Zheng QF, Tu WQ, Xing LD, Liao YH, Xu MQ, Huang QM, Cao GZ, Li WS (2018) Mechanism of cycling degradation and strategy to stabilize a nickel-rich cathode. J Mater Chem A 6(33):16149–16163
Chen T, Li X, Wang H, Yan XX, Wang L, Deng BW, Ge WJ, Qu MZ (2018) The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. J Power Sources 374:1–11
Liu BS, Sui XL, Zhang SH, Yu FD, Xue Y, Zhang Y, Zhou YX, Wang ZB (2018) Investigation on electrochemical performance of LiNi0.8Co0.15Al0.05O2 coated by heterogeneous layer of TiO2. J Alloys Compd 739:961–971
Liu WM, Guo HH, Qin ML, Deng JY, Xu L, Yi S, Hong TL (2018) Effect of voltage range and BiPO4 coating on the electrochemical properties of LiNi0.8Co−0.15Al0.05O2. ChemistrySelect 3(26):7660–7666
Liu ZH, Wang Z, Lu TZ, Dai PP, Gao P, Zhu YM (2018) Modification of LiNi0.8Co0.15Al0.05O2 using nanoscale carbon coating. J Alloys Compd 763:701–710
Chen JC, Zhu L, Jia D, Jiang XB, Wu YM, Hao QL, Xia XF, Ouyang Y, Peng LM, Tang WP, Liu T (2019) LiNi0.8Co0.15Al0.05O2 cathodes exhibiting improved capacity retention and thermal stability due to a lithium iron phosphate coating. Electrochim Acta 312:179–187
Liang LW, Sun X, Wu C, Hou LR, Sun JF, Zhang XG, Yuan CZ (2018) Nasicon-type surface functional modification in core shell LiNi(0.5)Mno(3)Co(0.2)O(2)@NaTi2(PO4)(3) cathode enhances its high-voltage cycling stability and rate capacity toward Li-ion batteries. ACS Appl Mater Interfaces 10(6):5498–5510
Lu JJ, Li WL, Shen C, Tang DM, Dai LX, Diao GW, Chen M (2019) Nano-scale hollow structure carbon-coated LiFePO4 as cathode material for lithium ion battery. Ionics 25(9):4075–4082
Zhao JK, Wang ZX, Guo HJ, Li XH (2017) Enhanced electrochemical properties of LiNiO2-based cathode materials by nanoscale manganese carbonate treatment. Appl Surf Sci 403:426–434
Huang YQ, Huang YH, Hu XL (2017) Enhanced electrochemical performance of LiNi0.8Co0.15Al0.05O2 by nanoscale surface modification with Co3O4. Electrochim. Acta 231:294–299
Ni LB, Zhao GJ, Wang YT, Wu Z, Wang W, Liao YY, Yang G, Diao GW (2017) Coaxial carbon/MnO2 hollow nanofibers as sulfur hosts for high-performance lithium–sulfur batteries. Chem Asian J 12(24):3128–3134
Ao X, Jiang JJ, Ruan YJ, Li ZS, Zhang Y, Sun JW, Wang CD (2017) Honeycomb-inspired design of ultrafine SnO2@C nanospheres embedded in carbon film as anode materials for high performance lithium- and sodium-ion battery. J Power Sources 359:340–348
Park KJ, Choi MJ, Maglia F, Kim SJ, Kim KH, Yoon CS, Sun YK (2018) High-capacity concentration gradient LiNi0.865Co0.120Al0.015O−2 cathode for lithium-ion batteries. Adv Energy Mater 8(19):10. https://doi.org/10.1002/aenm.201703612
Ramana CV, Zaghib K, Julien CM (2007) Pulsed-laser deposited LiNi0.8Co0.15Al0.05O2 thin films for application in microbatteries. Appl Phys Lett 90(2):3. https://doi.org/10.1063/1.2430933
Zhu L, Liu Y, Wu WY, Wu XW, Tang WP, Wu YP (2015) Surface fluorinated LiNi0.8Co0.15Al0.05O2 as a positive electrode material for lithium ion batteries. J Mater Chem A 3(29):15156–15162
Li XL, Liang M, Sheng J, Song DW, Zhang HZ, Shi XX, Zhang LQ (2019) Constructing double buffer layers to boost electrochemical performances of NCA cathode for ASSLB. Energy Storage Mater 18:100–106
Luo ZY, Zhang H, Yu L, Huang DH, Shen JQ (2019) Improving long-term cyclic performance of LiNi0.8Co0.15Al0.05O2 cathode by introducing a film forming additive. J Electroanal Chem 833:520–526
Meng HJ, Zhou PF, Zhang Z, Tao ZL, Chen J (2017) Preparation and characterization of LiNi0.8Co0.15Al0.05O2 with high cycling stability by using AlO2-as Al source. Ceram Int 43(4):3885–3892
Qiu ZP, Zhang YJ, Dong P, Wang D, Xia SB (2017) A ternary oxide precursor with trigonal structure for synthesis of LiNi0.8Co0.15Al0.05O2 cathode material. J Solid State Electrochem 21(10):3037–3046
Tang D, Zhao R, Shen C, Han Y, Wu X, Wu H, Diao G, Chen M (2019) High electrocatalytic performance of bimetallic sulfides dodecahedral nanocages (CoxM1−x)9S8/M/N–C (M = Ni, Cu) for triiodide reduction reaction and oxygen evolution reaction. Electrochim Acta 324:134888. https://doi.org/10.1016/j.electacta.2019.134888
Xie HB, Hu GR, Du K, Peng ZD, Cao YB (2016) An improved continuous co-precipitation method to synthesize LiNi0.8Co0.15Al0.05O2 cathode material. J Alloys Compd 666:84–87
Purwanto A, Yudha CS, Ubaidillah U, Widiyandari H, Ogi T, Haerudin H (2018) NCA cathode material: synthesis methods and performance enhancement efforts. Mater Res Express 5(12):22. https://doi.org/10.1088/2053-1591/aae167
Zhao JK, Wang ZX, Wang JX, Guo HJ, Li XH, Gui WH, Chen N, Yan GC (2018) Anchoring K+ in Li+ sites of LiNi0.8Co0.15Al0.05O2 cathode material to suppress its structural degradation during high-voltage cycling. Energy Technol 6(12):2358–2366
Li WD, Liu XM, Celio H, Smith P, Dolocan A, Chi MF, Manthiram A (2018) Mn versus Al in layered oxide cathodes in lithium-ion batteries: a comprehensive evaluation on long-term cyclability. Adv Energy Mater 8(15):11. https://doi.org/10.1002/aenm.201703154
Li X, Ge WJ, Wang H, Yan XX, Deng BW, Chen T, Qu MZ (2017) Enhancing cycle stability and storage property of LiNi0.8Co0.15Al0.05O2 by using fast cooling method. Electrochim Acta 227:225–234
Chen YJ, Li P, Zhao SJ, Zhuang Y, Zhao SY, Zhou Q, Zheng JW (2017) Influence of integrated microstructure on the performance of LiNi0.8Co0.15Al0.05O2 as a cathodic material for lithium ion batteries. RSC Adv 7(46):29233–29239
Acknowledgements
The funding support from the National Natural Science Foundation of China (Grant No. 21773203), the “Qinglan project” of Jiangsu Province (2018-12) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, X., Lu, J., Han, Y. et al. Template-assisted synthesis of LiNi0.8Co0.15Al0.05O2 hollow nanospheres as cathode material for lithium ion batteries. J Mater Sci 55, 9493–9503 (2020). https://doi.org/10.1007/s10853-020-04627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04627-1