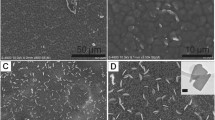
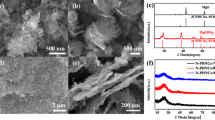
Abstract
Exploiting various nanocarbons for supercapacitors with excellent energy retention under ultrahigh power output in high-voltage ionic liquid electrolytes possesses enormous potential for next-generation supercapacitor applications. To address the poor rate capability of the carbon electrodes in ionic liquid electrolytes, we develop an efficient strategy toward porous nanocarbons with controllable microstructure that fully solves this bottleneck to gain an outstanding capacitive performance. We found the introduction of laser-induced graphene and Mg as the additives can serve multiple roles not only as the morphology modifier agents but also as the template for the developing rich mesopores. In particular, a curved nanosheet network-like porous carbons constructed by nanocages with ultrahigh specific surface area and considerable amount of mesopores can be rationally obtained. When served as an electrode for ionic liquid-based supercapacitor, it can display an impressive specific capacitance of 201.1 F/g at 1 A/g, superb rate performance (71% capacitance retained at 100 A/g) and encouraging energy density up to 60.76 Wh/kg at a recording power density of 87.5 kW/kg, which is comparable with the best results reported for carbon-based supercapacitors in ionic liquid electrolyte reported so far.







Similar content being viewed by others
References
Wang GP, Zhang L, Zhang JJ (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41(2):797–828
Chen XL, Paul R, Dai LM (2017) Carbon-based supercapacitors for efficient energy storage. Natl Sci Rev 4(3):453–489
Choudhary N, Li C, Moore J, Nagaiah N, Zhai L, Jung Y, Thomas J (2017) Asymmetric supercapacitor electrodes and devices. Adv Mater 29(21):201605336
Wu Z, Li L, Yan JM, Zhang XB (2017) Materials design and system construction for conventional and new-concept supercapacitors. Adv Sci 4(6):1600382
Poonam SK, Arora A, Tripathi SK (2019) Review of supercapacitors: materials and devices. J Energy Storage 21:801–825
Chmiola J, Yushin G, Gogotsi Y, Portet C, Simon P, Taberna PL (2006) Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313(5794):1760–1763
Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. J Power Sources 157(1):11–27
Yan J, Wang Q, Wei T, Fan ZJ (2014) Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv Energy Mater 4(4):1300816
Assresahegn BD, Bélanger D (2015) Multifunctional carbon for electrochemical double-layer capacitors. Adv Funct Mater 25(43):6775–6785
Sheberla D, Bachman JC, Elias JS, Sun CJ, Shao-Horn Y, Dinca M (2017) Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat Mater 16(2):220–224
Wang DW, Fang GL, Xue T, Ma JF, Geng GH (2016) A melt route for the synthesis of activated carbon derived from carton box for high performance symmetric supercapacitor applications. J Power Sources 307:401–409
Wang Y, Song Y, Xia Y (2016) Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem Soc Rev 45(21):5925–5950
Prehal C, Koczwara C, Jackel N, Schreiber A, Burian M, Amenitsch H, Hartmann MA, Presser V, Paris O (2017) Quantification of ion confinement and desolvation in nanoporous carbon supercapacitors with modelling and in situ X-ray scattering. Nat Energy 2(3):16215
Wei X, Jiang X, Wei J, Gao S (2016) Functional groups and pore size distribution do matter to hierarchically porous carbons as high-rate-performance supercapacitors. Chem Mater 28(2):445–458
Wang DW, Liu SJ, Fang GL, Geng GH, Ma JF (2016) From trash to treasure: direct transformation of onion husks into three-dimensional interconnected porous carbon frameworks for high-performance supercapacitors in organic electrolyte. Electrochim Acta 216:405–411
Salanne M, Rotenberg B, Naoi K, Kaneko K, Taberna PL, Grey CP, Dunn B, Simon P (2016) Efficient storage mechanisms for building better supercapacitors. Nat Energy 1:16070
Gonzalez A, Goikolea E, Andoni Barrena J, Mysyk R (2016) Review on supercapacitors: technologies and materials. Renew Sustain Energy Rev 58:1189–1206
Yang Z, Ren J, Zhang Z, Chen X, Guan G, Qiu L, Zhang Y, Peng H (2015) Recent advancement of nanostructured carbon for energy applications. Chem Rev 115(11):5159–5223
Ni J, Li Y (2016) Carbon nanomaterials in different dimensions for electrochemical energy storage. Adv Energy Mater 6(17):1600278
Liu CF, Liu YC, Yi TY, Hu CC (2019) Carbon materials for high-voltage supercapacitors. Carbon 145:529–548
MacFarlane DR, Tachikawa N, Forsyth M, Pringle JM, Howlett PC, Elliott GD, Davis JH, Watanabe M, Simon P, Angell CA (2013) Energy applications of ionic liquids. Energy Environ Sci 7(1):232–250
Sillars FB, Fletcher SI, Mirzaeian M, Hall PJ (2012) Variation of electrochemical capacitor performance with room temperature ionic liquid electrolyte viscosity and ion size. Phys Chem Chem Phys 14(17):6094–6100
Wang DW, Nai JW, Li H, Xu L, Wang YT (2019) A robust strategy for the general synthesis of hierarchical carbons constructed by nanosheets and their application in high performance supercapacitor in ionic liquid electrolyte. Carbon 141:40–49
Kado Y, Soneda Y, Hatori H, Kodama M (2019) Advanced carbon electrode for electrochemical capacitors. J Solid State Electrochem 23(4):1061–1081
Béguin F, Presser V, Balducci A, Frackowiak E (2014) Carbons and electrolytes for advanced supercapacitors. Adv Mater 26(14):2219–2251
Fic K, Frackowiak E, Beguin F (2012) Unusual energy enhancement in carbon-based electrochemical capacitors. J Mater Chem 22(46):24213–24223
Fic K, Lota G, Meller M, Frackowiak E (2012) Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ Sci 5(2):5842–5850
Wang DW, Wang YT, Liu HW, Xu W, Xu L (2018) Unusual carbon nanomesh constructed by interconnected carbon nanocages for ionic liquid-based supercapacitor with superior rate capability. Chem Eng J 342:474–483
Schutjajew K, Yan RY, Antonietti M, Roth C, Oschatz M (2019) Effects of carbon pore size on the contribution of ionic liquid electrolyte phase transitions to energy storage in supercapacitors. Front Mater 6:65
Yan RY, Antonietti M, Oschatz M (2018) Toward the experimental understanding of the energy storage mechanism and ion dynamics in ionic liquid based supercapacitors. Adv Energy Mater 8(18):1800026
Vatamanu J, Hu ZZ, Bedrov D, Perez C, Gogotsi Y (2013) Increasing energy storage in electrochemical capacitors with ionic liquid electrolytes and nanostructured carbon electrodes. J Phys Chem Lett 4(17):2829–2837
Zhao X, Chen HL, Kong FG, Zhang YJ, Wang SJ, Liu SX, Lucia LA, Fatehi P, Pang H (2019) Fabrication, characteristics and applications of carbon materials with different morphologies and porous structures produced from wood liquefaction: a review. Chem Eng J 364:226–243
Sun HT, Zhu J, Baumann D, Peng LL, Xu YX, Shakir I, Huang Y, Duan XF (2019) Hierarchical 3D electrodes for electrochemical energy storage. Nat Rev Mater 4(1):45–60
Mehtab T, Yasin G, Arif M, Shakeel M, Korai RM, Nadeem M, Muhammad N, Lu X (2019) Metal–organic frameworks for energy storage devices: batteries and supercapacitors. J Energy Storage 21:632–646
Kumar Y, Rawal S, Joshi B, Hashmi SA (2019) Background, fundamental understanding and progress in electrochemical capacitors. J Solid State Electrochem 23(3):667–692
Ruoff RS (2018) A perspective on objectives for carbon science. Carbon 132:802
Raza W, Ali FZ, Raza N, Luo YW, Kim KH, Yang JH, Kumar S, Mehmood A, Kwon EE (2018) Recent advancements in supercapacitor technology. Nano Energy 52:441–473
Wang DW, Min YG, Yu YH, Peng B (2014) Laser induced self-propagating reduction and exfoliation of graphite oxide as an electrode material for supercapacitors. Electrochim Acta 141:271–278
Wang JC, Kaskel S (2012) KOH activation of carbon-based materials for energy storage. J Mater Chem 22(45):23710–23725
He X, Zhang N, Shao X, Wu M, Yu M, Qiu J (2016) A layered-template-nanospace-confinement strategy for production of corrugated graphene nanosheets from petroleum pitch for supercapacitors. Chem Eng J 297:121–127
Dyjak S, Kicinski W, Norek M, Huczko A, Labedz O, Budner S, Polanski M (2016) Hierarchical, nanoporous graphenic carbon materials through an instant, self-sustaining magnesiothermic reduction. Carbon 96:937–946
Pimenta MA, Dresselhaus G, Dresselhaus MS, Cancado LG, Jorio A, Saito R (2007) Studying disorder in graphite-based systems by Raman spectroscopy. Phys Chem Chem Phys 9(11):1276–1291
Azaïs P, Duclaux L, Florian P, Massiot D, Lillo-Rodenas MA, Linares-Solano A, Peres JP, Jehoulet C, Béguin F (2007) Causes of supercapacitors ageing in organic electrolyte. J Power Sources 171(2):1046–1053
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem 87(9–10):1051–1069
Segalini J, Daffos B, Taberna PL, Gogotsi Y, Simon P (2010) Qualitative electrochemical impedance spectroscopy study of ion transport into sub-nanometer carbon pores in electrochemical double layer capacitor electrodes. Electrochim Acta 55(25):7489–7494
Chen HJ, Chen J, Chen DM, Wei HM, Liu P, Wei W, Lin HL, Han S (2019) Nitrogen- and oxygen-rich dual-decorated carbon materials with porosity for high-performance supercapacitors. J Mater Sci 54(7):5625–5640. https://doi.org/10.1007/s10853-018-2993-x
Thubsuang U, Laebang S, Manmuanpom N, Wongkasemjit S, Chaisuwan T (2017) Tuning pore characteristics of porous carbon monoliths prepared from rubber wood waste treated with H3PO4 or NaOH and their potential as supercapacitor electrode materials. J Mater Sci 52(11):6837–6855. https://doi.org/10.1007/s10853-017-0922-z
Fuertes AB, Sevilla M (2015) High-surface area carbons from renewable sources with a bimodal micro-mesoporosity for high-performance ionic liquid-based supercapacitors. Carbon 94:41–52
Shao Q, Tang J, Lin Y, Li J, Qin F, Yuan J, Qin L (2015) Carbon nanotube spaced graphene aerogels with enhanced capacitance in aqueous and ionic liquid electrolytes. J Power Sources 278:751–759
Yang CH, Huang PL, Luo XF, Wang CH, Li C, Wu YH, Chang JK (2015) Holey graphene nanosheets with surface functional groups as high-performance supercapacitors in ionic liquid electrolyte. Chemsuschem 8(10):1779–1786
Guo NN, Li M, Wang Y, Sun XK, Wang F, Yang R (2016) Soybean root-derived hierarchical porous carbon as electrode material for high-performance supercapacitors in ionic liquids. ACS Appl Mater Interfaces 8(49):33626–33634
Wang XH, Zhou HT, Lou FL, Li YH, Buan MEM, Duan XZ, Walmsley JC, Sheridan E, Chen D (2016) Boosted supercapacitive energy with high rate capability of acarbon framework with hierarchical pore structure in an ionic liquid. Chemsuschem 9(21):3093–3101
Gao BF, Zhou HT, Yang JH (2017) One-step preparation of nitrogen-doped graphene nanosheets for high-performance supercapacitors. Appl Surf Sci 409:350–357
Guo NN, Li M, Sun XK, Wang F, Yang R (2017) Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities. Green Chem 19(11):2595–2602
Hao EC, Liu W, Liu S, Zhang Y, Wang HL, Chen SG, Cheng FL, Zhao SP, Yang HZ (2017) Rich sulfur doped porous carbon materials derived from ginkgo leaves for multiple electrochemical energy storage devices. J Mater Chem A 5(5):2204–2214
Niu J, Shao R, Liang JJ, Dou ML, Li ZL, Huang YQ, Wang F (2017) Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 36:322–330
Su H, Zhang HT, Liu FY, Chun FJ, Zhang BB, Chu X, Huang HC, Deng WL, Gu BN, Zhang HP, Zheng XT, Zhu MH, Yang WQ (2017) High power supercapacitors based on hierarchically porous sheet-like nanocarbons with ionic liquid electrolytes. Chem Eng J 322:73–81
Tian WQ, Gao QM, Tan YL, Li ZY (2017) Unusual interconnected graphitized carbon nanosheets as the electrode of high-rate ionic liquid-based supercapacitor. Carbon 119:287–295
Chen YJ, Liu ZE, Sun L, Lu ZW, Zhuo KL (2018) Nitrogen and sulfur co-doped porous graphene aerogel as an efficient electrode material for high performance supercapacitor in ionic liquid electrolyte. J Power Sources 390:215–223
Kannappan S, Yang H, Kaliyappan K, Manian RK, Pandian AS, Lee YS, Jang JH, Lu W (2018) Thiolated-graphene-based supercapacitors with high energy density and stable cycling performance. Carbon 134:326–333
Sun JT, Niu J, Liu MY, Ji J, Dou ML, Wang F (2018) Biomass-derived nitrogen-doped porous carbons with tailored hierarchical porosity and high specific surface area for high energy and power density supercapacitors. Appl Surf Sci 427:807–813
Thangavel R, Kannan AG, Ponraj R, Thangavel V, Kim DW, Lee YS (2018) High-energy green supercapacitor driven by ionic liquid electrolytes as an ultra-high stable next-generation energy storage device. J Power Sources 383:102–109
Wang DW, Nai JW, Xu L, Sun T (2019) A potassium formate activation strategy for the synthesis of ultrathin graphene-like porous carbon nanosheets for advanced supercapacitor applications. ACS Sustain Chem Eng 7(23):18901–18911
Liang JX, Xiao ZC, Gao Y, Xu XH, Kong DB, Wagner M, Zhi LJ (2019) Ionothermal strategy towards template-free hierarchical porous carbons for supercapacitive energy storage. Carbon 143:487–493
Wang DW, Xu L, Nai JW, Sun T (2019) A versatile co-activation strategy towards porous carbon nanosheets for high performance ionic liquid based supercapacitor applications. J Alloys Compd 786:109–117
Momodu D, Sylla NF, Mutuma B, Bello A, Masikhwa T, Lindberg S, Matic A, Manyala N (2019) Stable ionic-liquid-based symmetric supercapacitors from Capsicum seed-porous carbons. J Electroanal Chem 838:119–128
Acknowledgements
The authors are grateful to the financial supports from the National Natural Science Foundation of China (Grant No. 51762001), CAS "Light of West China" Program (Grant No. XAB2017AW07), Natural Science Foundation of Ningxia (Grant No. 2018AAC03108), and Key Scientific Research Projects at North Minzu University (Grant No. ZDZX201802).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file 1 (MP4 10218 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Lu, Z. & Xu, L. Microstructure design of porous nanocarbons for ultrahigh-energy and power density supercapacitors in ionic liquid electrolyte. J Mater Sci 55, 7477–7491 (2020). https://doi.org/10.1007/s10853-020-04538-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04538-1