Abstract
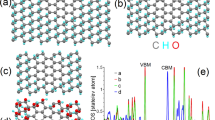
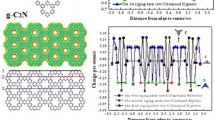
The graphitic carbon nitride (g-C3N4) nanodots (CN-dots) exhibit properties different from those of g-C3N4 crystal. However, the electronic structure of g-C3N4 nanodots, which determines their properties intrinsically, has not been explored comprehensively. Herein, the many-body Green’s function theory is used to analyze the electronic and optical properties of CN-dots; and the effects of size, shape, and functional group on properties were systematically investigated. The large size and the nonlinear shape are effective means to decrease electronic band gap. The increase in the functional group –CHO can make the complex composed of 1D g-C3N4 and 2D g-C3N4 change from type I to type II heterojunction. Different functional groups are related to the absorption edge of CN-dots, while have little effect on the electron–hole recombination rate. These results can provide theoretical support for modifying the properties of CN-dots and further designing CN-dots-based functional materials.






Similar content being viewed by others
References
Ong W, Tan L, Ng YH, Yong S, Chai S (2016) Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem Rev 116(12):7159–7329
Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM, Domen K, Antonietti M (2009) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8(1):76–80
Xu C, Han Q, Zhao Y, Wang L, Li Y, Qu L (2015) Sulfur-doped graphitic carbon nitride decorated with graphene quantum dots for an efficient metal-free electrocatalyst. J Mater Chem 3(5):1841–1846
She X, Liu L, Ji H, Mo Z, Li Y, Huang L, Du D, Hui X, Li H (2016) Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl Catal B. https://doi.org/10.1016/j.apcatb.2015.12.046
Li J, Shen B, Hong Z, Lin B, Gao B, Chen Y (2012) A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem Commun 48(98):12017–12019
Xu C-Q, Li K, Zhang W-D (2017) Enhancing visible light photocatalytic activity of nitrogen-deficient g-C3N4 via thermal polymerization of acetic acid-treated melamine. J Colloid Interface Sci 495:27–36
Liu J, Liu Y, Liu N, Han Y, Zhang X, Huang H, Lifshitz Y, Lee S, Zhong J, Kang Z (2015) Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347(6225):970–974
Huang Z, Song J, Pan L, Wang Z, Zhang X, Zou J, Mi W, Zhang X, Wang L (2015) Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 12:646–656
Lin Z, Wang X (2013) Nanostructure engineering and doping of conjugated carbon nitride semiconductors for hydrogen photosynthesis. Angew Chem 52(6):1735–1738
Feng J, Ma H, Chen T, Liu C, Ma Y (2018) Passivated codoping can improve the solar-to-hydrogen efficiency of graphitic carbon nitride. J Phys Chem C 122(13):7296–7302
Gao G, Jiao Y, Ma F, Jiao Y, Waclawik ER, Du A (2015) Carbon nanodot decorated graphitic carbon nitride: new insights into the enhanced photocatalytic water splitting from ab initio studies. Phys Chem Chem Phys 17(46):31140–31144
Ma Z, Sa R, Li Q, Wu K (2016) Interfacial electronic structure and charge transfer of hybrid graphene quantum dot and graphitic carbon nitride nanocomposites: insights into high efficiency for photocatalytic solar water splitting. Phys Chem Chem Phys 18(2):1050–1058
Feng J, Liu G, Yuan S, Ma Y (2017) Influence of functional groups on water splitting in carbon nanodot and graphitic carbon nitride composites: a theoretical mechanism study. Phys Chem Chem Phys 19(7):4997–5003
Mao J, Peng T, Zhang X, Li K, Ye L, Zan L (2013) Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal Sci Technol 3(5):1253–1260
Jiao Y, Zheng Y, Chen P, Jaroniec M, Qiao S (2017) Molecular scaffolding strategy with synergistic active centers to facilitate electrocatalytic CO2 reduction to hydrocarbon/alcohol. J Am Chem Soc 139(49):18093–18100
Shi H, Chen G, Zhang C, Zou Z (2014) Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel. ACS Catal 4(10):3637–3643
Xu D, Cheng B, Wang W, Jiang C, Yu J (2018) Ag2CrO4/g-C3N4/graphene oxide ternary nanocomposite Z-scheme photocatalyst with enhanced CO2 reduction activity. Appl Catal B 231:368–380
Wang K, Li Q, Liu B, Cheng B, Ho W, Yu J (2015) Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl Catal B 176:44–52
Wang Y, Zhang J, Wang X, Antonietti M, Li H (2010) Boron- and fluorine-containing mesoporous carbon nitride polymers: metal-free catalysts for cyclohexane oxidation. Angew Chem 49(19):3356–3359
Jie L, Chuanbao C, Hesun Z (2007) Synthesis and characterization of graphite-like carbon nitride nanobelts and nanotubes. Nanotechnology 18(11):115605
Gao X, Jiao X, Zhang L, Zhu W, Xu X, Ma H, Chen T (2015) Cosolvent-free nanocasting synthesis of ordered mesoporous g-C3N4 and its remarkable photocatalytic activity for methyl orange degradation. RSC Adv 5(94):76963–76972. https://doi.org/10.1039/C5RA13438B
Kessler FK, Zheng Y, Schwarz D, Merschjann C, Schnick W, Wang X, Bojdys MJ (2017) Functional carbon nitride materials—design strategies for electrochemical devices. Nat Rev Mater 2:17030. https://doi.org/10.1038/natrevmats.2017.30
Tahir M, Cao C, Butt FK, Idrees F, Mahmood N, Ali Z, Aslam I, Tanveer M, Rizwan M, Mahmood T (2013) Tubular graphitic-C3N4: a prospective material for energy storage and green photocatalysis. J Mater Chem 1(44):13949–13955
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126(40):12736–12737
Li H, Kang Z, Liu Y, Lee ST (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22(46):24230–24253
Liu Q, Chen T, Guo Y, Zhang Z, Fang X (2016) Ultrathin g-C3N4 nanosheets coupled with carbon nanodots as 2D/0D composites for efficient photocatalytic H2 evolution. Appl Catal B 193:248–258
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49(38):6726–6744
Cao L, Sahu S, Anilkumar P, Bunker CE, Xu J, Fernando KS, Wang P, Guliants EA, Tackett KN, Sun Y-P (2011) Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J Am Chem Soc 133(13):4754–4757
Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CHA, Yang X, Lee ST (2010) Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew Chem Int Ed 49(26):4430–4434
Kang Z, Liu Y, Lee S-T (2011) Small-sized silicon nanoparticles: new nanolights and nanocatalysts. Nanoscale 3(3):777–791
Ding Z, Quinn BM, Haram SK, Pell LE, Korgel BA, Bard AJ (2002) Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science 296(5571):1293–1297
Ming H, Yan Y, Ming J, Li X, Zhou Q, Huang H, Zheng J (2014) Porous TiO2 nanoribbons and TiO2 nanoribbon/carbon dot composites for enhanced Li-ion storage. RSC Adv 4(25):12971–12976
Mo R, Lei Z, Sun K, Rooney D (2014) Facile synthesis of anatase TiO2 quantum-dot/graphene-nanosheet composites with enhanced electrochemical performance for lithium-ion batteries. Adv Mater 26(13):2084–2088
Wang W, Jimmy CY, Shen Z, Chan DK, Gu T (2014) g-C3N4 quantum dots: direct synthesis, upconversion properties and photocatalytic application. Chem Commun 50(70):10148–10150
Wang X, Sun G, Li N, Chen P (2016) Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem Soc Rev 45(8):2239–2262
Corp KL, Schlenker CW (2017) Ultrafast spectroscopy reveals electron-transfer cascade that improves hydrogen evolution with carbon nitride photocatalysts. J Am Chem Soc 139(23):7904–7912
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Perdew JP, Ernzerhof M, Burke K (1996) Rationale for mixing exact exchange with density functional approximations. J Chem Phys 105(22):9982–9985
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186. https://doi.org/10.1103/PhysRevB.54.11169
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799
Rohlfing M, Krüger P, Pollmann J (1995) Efficient scheme for GW quasiparticle band-structure calculations with applications to bulk Si and to the Si (001)-(2 × 1) surface. Phys Rev B 52(3):1905
Rohlfing M, Louie SG (2000) Electron-hole excitations and optical spectra from first principles. Phys Rev B 62(8):4927
Eda G, Lin YY, Mattevi C, Yamaguchi H, Chen HA, Chen IS, Chen CW, Chhowalla M (2010) Blue photoluminescence from chemically derived graphene oxide. Adv Mater 22(4):505–509
Frisch MJ, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Fox DJ (2015) Gaussian 09, revision A. 02. Gaussian Inc, Wallingford
Hu W, Lin L, Zhang R, Yang C, Yang J (2017) Highly efficient photocatalytic water splitting over edge-modified phosphorene nanoribbons. J Am Chem Soc 139(43):15429–15436
Zhai S, Guo P, Zheng J, Zhao P, Suo B, Wan Y (2018) Density functional theory study on the stability, electronic structure and absorption spectrum of small size g-C3N4 quantum dots. Comput Mater Sci 148:149–156
Liu G, Niu P, Sun C, Smith SC, Chen Z, Lu GQ, Cheng H-M (2010) Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J Am Chem Soc 132(33):11642–11648
Acknowledgements
The authors greatly acknowledge the financial support from the National Natural Science Foundation of China (NSFC) (G. Nos. 21903048, 21873055, 21833004, 21573131 and 21433006). We are also thankful to the High Performance Computing Center of Qufu Normal University for the use of computational resources.
Author information
Authors and Affiliations
Contributions
Jin Feng and Dapeng Zhang completed major experimental design, calculations, data analysis and article writing; Jiawei Li calculated the calculation of adsorption energy; Siwei Bi and Yuchen Ma helped to modify the experimental ideas and language of manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, J., Zhang, D., Li, J. et al. Graphitic carbon nitride nanodots: electronic structure and its influence factors. J Mater Sci 55, 5488–5498 (2020). https://doi.org/10.1007/s10853-020-04396-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04396-x