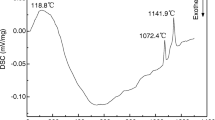
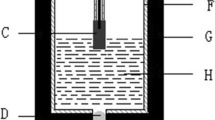
Abstract
A new type of Fe–Ni ceramet inert anode with the NiO contents of 0, 10, 20 and 30 wt% for aluminum electrolysis was prepared by the powder metallurgy process. The effects of NiO addition on the high-temperature oxidation and corrosion behaviors of the ceramet had been investigated by the thermogravimetric analysis and electrolysis, respectively. The results indicated that the pre-existing oxide phase shortened the time for formation of the oxide scale on the sample, which promoted the formation of continuous dense scale with the good adhesion to substrate materials during high-temperature oxidation. Besides, the appearance of NiO at the grain boundary hindered the oxidation of the metal phase, which avoids the peeling phenomenon. As a result, the addition of NiO in the Fe–Ni alloy played an important role in hindering the oxidation process, which greatly improved the anti-oxidant properties. Compared with the alloy anode, additionally, the total impurity content in the melted aluminum electrolyte was 0.28 mass% for 100 h of electrolysis at 20 A. It indicated that the addition of NiO improved the corrosion resistance of Fe–Ni ceramet anode significantly. Therefore, the Fe–Ni ceramet anode may be the promising inert anode materials for the aluminum electrolysis.








Similar content being viewed by others
References
Benedyk JC (2001) Status report on inert anode technology for primary aluminum. Light Met Age 59(2):36–37
Nora D (2001) Inert anodes are knocking at the door of aluminium producers. In: CRU annual meeting, vol 26, London
Kvande H, Haupin W (2001) Inert anodes for AI smelters: energy balances and environmental impact. JOM 53:29–33
Sadoway DR (2001) Inert anodes for the Hall–Héroult cell: the ultimate materials challenge. JOM 53:34–35
Feng LC, Shao WZ, Zhen L, Xie N, Ivanov VV (2007) Cu2O/Cu cermet as a candidate inert anode for Al production. Int J Appl Ceram Technol 4:453–462
Wei W, Geng SJ, Xie DB, Wang FH (2019) High temperature oxidation and corrosion behaviours of Ni–Fe–Cr alloys as inert anode for aluminum electrolysis. Corros Sci 157:382–391
Cao D, Ma YF, Shi ZN, Xu L, Hu XW, Wang ZW (2019) Corrosion behavior of Fe–Ni alloys in molten KF–AlF3–Al2O3 salts at 700 °C. Corros Sci 156:32–43
Chapman V, Welch BJ, Skyllas-Kazacos M (2011) Anodic behaviour of oxidised Ni–Fe alloys in cryolite-alumina melts. Electrochim Acta 56(3):1227–1238
Helle S, Pedron M, Assouli B, Davis B, Guay D, Roué L (2010) Structure and high-temperature oxidation behaviour of Cu–Ni–Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminium electrolysis. Corros Sci 52(10):3348–3355
Sadoway DR (1990) A materials systems approach to selection and testing of non-consumable anodes for the Hall cell. Light Met TMS, 403–407
Chapman V, Welch BJ, Skyllas-Kazacos M (2011) High temperature oxidation behaviour of Ni–Fe–Co anodes for aluminium electrolysis. Corros Sci 53:2815–2825
Khramov AP, Kovrov VA, Zaikov YP, Chumarev VM (2013) Anodic behaviour of the Cu82Al8Ni5Fe5 alloy in low-temperature aluminium electrolysis. Corros Sci 70(12):194–202
Gavrilova E, Goupil G, Davis B, Guaya D, Rouéa L (2015) On the key role of Cu on the oxidation behavior of Cu–Ni–Fe based anodes for Al electrolysis. Corros Sci 101:105–113
Haugsrud R, Norby T, Kofstad P (2001) High-temperature oxidation of Cu-30 wt% Ni-15 wt% Fe. Corros Sci 43:283–299
Yin HY, Gao LL, Zhu H, Mao XH, Gan FX, Wang DH (2011) On the development of metallic inert anode for molten CaCl2–CaO system. Electrochim Acta 56:3296–3302
Haugsrud R (2000) High-temperature oxidation of Cu-10 wt% Ni and Cu-15 wt% Ni at 900–1050 °C. Corros Sci 42:383–399
Summerfelt SR, Carter CB (1992) Dissolution of NiFe2O4 particles in a NiO substrate. Acta Metall Sin 40(10):2799–2804
Lin QQ, Long YP, Dong WZ (2014) Effect of Y2O3 doped on high-temperature oxidation performance of 17Ni/(NiFe2O4–10NiO) cermet. Bull Ceram Soc 33(4):890–894
Tucker MC, Lau GY, Jacobson CP, Visco SJ, DeJonghe LC (2010) Cu–YSZ cermet solid oxide fuel anode prepared by high-temperature sintering. J Power Sources 195:3119–3123
Goupil G, Helle S, Davis B, Guay D, Roué L (2013) Anodic behavior of mechanically alloyed Cu–Ni–Fe and Cu–Ni–Fe–O electrodes for aluminum electrolysis in low-temperature KF–AlF3 electrolyte. Electrochim Acta 112:176–182
Dalvi AD, Smeltzer WW (1970) Thermodynamics of the iron–nickel–oxygen system at 1000 °C. J Electrochem Soc 117:1431–1436
Rhamdhani MA, Hayes PC, Jak E (2008) Subsolidus phase equilibria of the Fe–Ni–O system. Metall Mater Trans B 39(5):690–701
Acknowledgements
This work was supported in part by the financial support of the Key Scientific Research Projects of Higher Education Institutions in Henan Province (19B430013) and the Young Key Teachers Training Program of Henan Province Higher Education Institutions (2018GGJS090).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Wj., Wang, Y., Zhai, Hf. et al. Effect of NiO addition on the high-temperature oxidation and corrosion behaviors of Fe–Ni alloy as inert anode material for aluminum electrolysis. J Mater Sci 55, 4065–4072 (2020). https://doi.org/10.1007/s10853-019-04250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04250-9