Abstract
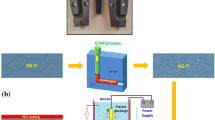
The nanostructured titanium (Ti) obtained by the equal-channel angular pressing (ECAP) has shown great promise as a biomedical implant material over the past few decades. The present work aims to investigate the effect of topographical changes caused by ECAP and piranha treatment (Tr) on the surface performance and biological properties of Ti for bone tissue engineering applications. The effects of dual treatments, i.e., ECAP and Tr, on Ti were systematically investigated by multiple characterization techniques, surface wettability, apatite-forming ability, cellular behavior, and antibacterial studies. We demonstrate that both ECAP and ECApTr samples possess desirable mechanical and physical properties and are biocompatible to cultured human fetal osteoblast (hFOB) cells. The potential of adhesion and proliferation of hFOB cells on ECAP and ECApTr samples was found to be superior to that of control unprocessed sample (annealed). Ti samples prepared by both methods showed excellent antimicrobial properties against clinical strains of the most common pathogenic bacteria causing orthopedic implant infections, Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa). This study supports the established claim about mechanical properties improvement by ultrafine refinement and further enhances the antibacterial properties when chemically etched with a piranha solution.










Similar content being viewed by others
References
Simchi A, Tamjid E, Pishbin F, Boccaccini AR (2011) Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed Nanotechnol Biol Med 7(1):22–39. https://doi.org/10.1016/j.nano.2010.10.005
Hasan J, Crawford RJ, Ivanova EP (2013) Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol 31(5):295–304. https://doi.org/10.1016/j.tibtech.2013.01.017
Leventhal GS (1951) Titanium, a metal for surgery. J Bone Joint Surg 33(2):473–474
Brånemark PI, Breine U, Johansson B, Roylance PJ, Röckert H, Yoffey JM (1964) Regeneration of bone marrow. Cells Tissues Organs 59(1–2):1–46. https://doi.org/10.1159/000142601
Long M, Rack HJ (1998) Titanium alloys in total joint replacement—a materials science perspective. Biomaterials 19(18):1621–1639. https://doi.org/10.1016/S0142-9612(97)00146-4
Niinomi M (2003) Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci Technol Adv Mater 4(5):445–454. https://doi.org/10.1016/j.stam.2003.09.002
Li Y, Yang C, Zhao H, Qu S, Li X, Li Y (2014) New developments of Ti-based alloys for biomedical applications. Materials 7(3):1709–1800. https://doi.org/10.3390/ma7031709
Morais LS, Serra GG, Albuquerque Palermo EF, Andrade LR, Müller CA, Meyers MA, Elias CN (2009) Systemic levels of metallic ions released from orthodontic mini-implants. Am J Orthod Dentofac Orthop 135(4):522–529. https://doi.org/10.1016/j.ajodo.2007.04.045
Okazaki Y, Gotoh E, Manabe T, Kobayashi K (2004) Comparison of metal concentrations in rat tibia tissues with various metallic implants. Biomaterials 25(28):5913–5920. https://doi.org/10.1016/j.biomaterials.2004.01.064
Shettlemore MG, Bundy KJ (1999) Toxicity measurement of orthopedic implant alloy degradation products using a bioluminescent bacterial assay. J Biomed Mater Res 45(4):395–403. https://doi.org/10.1002/(SICI)1097-4636(19990615)45:4%3c395:AID-JBM15%3e3.0.CO;2-H
Valiev RZ, Estrin Y, Horita Z, Langdon TG, Zechetbauer MJ, Zhu YT (2006) Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 58(4):33–39. https://doi.org/10.1007/s11837-006-0213-7
Estrin Y, Vinogradov A (2013) Extreme grain refinement by severe plastic deformation: a wealth of challenging science. Acta Mater 61(3):782–817. https://doi.org/10.1016/j.actamat.2012.10.038
Ratna Sunil B, Thirugnanam A, Chakkingal U, Sampath Kumar TS (2016) Nano and ultra fine grained metallic biomaterials by severe plastic deformation techniques. Mater Technol 31(13):743–755. https://doi.org/10.1080/10667857.2016.1249133
Misra RDK, Thein-Han WW, Pesacreta TC, Somani MC, Karjalainen LP (2010) Biological significance of nanograined/ultrafine-grained structures: interaction with fibroblasts. Acta Biomater 6(8):3339–3348. https://doi.org/10.1016/j.actbio.2010.01.034
Park J-W, Kim Y-J, Park CH, Lee D-H, Ko YG, Jang J-H, Lee CS (2009) Enhanced osteoblast response to an equal channel angular pressing-processed pure titanium substrate with microrough surface topography. Acta Biomater 5(8):3272–3280. https://doi.org/10.1016/j.actbio.2009.04.038
Tan AW, Pingguan-Murphy B, Ahmad R, Akbar SA (2012) Review of titania nanotubes: fabrication and cellular response. Ceram Int 38(6):4421–4435. https://doi.org/10.1016/j.ceramint.2012.03.002
Liu X, Chu PK, Ding C (2004) Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng R Rep 47(3):49–121. https://doi.org/10.1016/j.mser.2004.11.001
Lord MS, Modin C, Foss M, Duch M, Simmons A, Pedersen FS, Milthorpe BK, Besenbacher F (2006) Monitoring cell adhesion on tantalum and oxidised polystyrene using a quartz crystal microbalance with dissipation. Biomaterials 27(26):4529–4537. https://doi.org/10.1016/j.biomaterials.2006.04.006
Jojibabu P, Ratna Sunil B, Sampath Kumar TS, Chakkingal U, Nandakumar V, Doble M (2013) Wettability and in vitro bioactivity studies on titanium rods processed by equal channel angular pressing. Trans Indian Inst Met 66(4):299–304. https://doi.org/10.1007/s12666-013-0281-7
Variola F, Brunski J, Orsini G, de Oliveira PT, Wazen R, Nanci A (2011) Nanoscale surface modifications of medically-relevant metals: state-of-the art and perspectives. Nanoscale 3(2):335–353. https://doi.org/10.1039/c0nr00485e
de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A (2007) Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res Part A 80A(3):554–564. https://doi.org/10.1002/jbm.a.30955
Webster TJ, Ejiofor JU (2004) Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4 V, and CoCrMo. Biomaterials 25(19):4731–4739. https://doi.org/10.1016/j.biomaterials.2003.12.002
Popat KC, Leoni L, Grimes CA, Desai TA (2007) Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 28(21):3188–3197. https://doi.org/10.1016/j.biomaterials.2007.03.020
Curtis A, Wilkinson C (1997) Topographical control of cells. Biomaterials 18(24):1573–1583. https://doi.org/10.1016/S0142-9612(97)00144-0
Edwards KJ, Rutenberg AD (2001) Microbial response to surface microtopography: the role of metabolism in localized mineral dissolution. Chem Geol 180(1):19–32. https://doi.org/10.1016/S0009-2541(01)00303-5
Tambasco de Oliveira P, Nanci A (2004) Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials 25(3):403–413. https://doi.org/10.1016/S0142-9612(03)00539-8
Seddiki O, Harnagea C, Levesque L, Mantovani D, Rosei F (2014) Evidence of antibacterial activity on titanium surfaces through nanotextures. Appl Surf Sci 308:275–284. https://doi.org/10.1016/j.apsusc.2014.04.155
Sandeep Kranthi Kiran A, Sampath Kumar TS, Perumal G, Sanghavi R, Doble M, Ramakrishna S (2018) Dual nanofibrous bioactive coating and antimicrobial surface treatment for infection resistant titanium implants. Prog Org Coat 121:112–119. https://doi.org/10.1016/j.porgcoat.2018.04.028
Mishra A, Kad BK, Gregori F, Meyers MA (2007) Microstructural evolution in copper subjected to severe plastic deformation: experiments and analysis. Acta Mater 55(1):13–28. https://doi.org/10.1016/j.actamat.2006.07.008
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27(15):2907–2915. https://doi.org/10.1016/j.biomaterials.2006.01.017
Harris SA, Enger RJ, Riggs LB, Spelsberg TC (1995) Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res 10(2):178–186. https://doi.org/10.1002/jbmr.5650100203
Kim TN, Balakrishnan A, Lee BC, Kim WS, Dvorankova B, Smetana K, Park JK, Panigrahi BB (2007) In vitro fibroblast response to ultra fine grained titanium produced by a severe plastic deformation process. J Mater Sci Mater Med 19(2):553–557. https://doi.org/10.1007/s10856-007-3204-5
Bauer S, Schmuki P, von der Mark K, Park J (2013) Engineering biocompatible implant surfaces: Part I: materials and surfaces. Prog Mater Sci 58(3):261–326. https://doi.org/10.1016/j.pmatsci.2012.09.001
Simi VS, Rajendran N (2017) Influence of tunable diameter on the electrochemical behavior and antibacterial activity of titania nanotube arrays for biomedical applications. Mater Charact 129:67–79. https://doi.org/10.1016/j.matchar.2017.04.019
Attar H, Calin M, Zhang LC, Scudino S, Eckert J (2014) Manufacture by selective laser melting and mechanical behavior of commercially pure titanium. Mater Sci Eng A 593:170–177. https://doi.org/10.1016/j.msea.2013.11.038
Ketabchi A, Komm K, Miles-Rossouw M, Cassani DAD, Variola F (2014) Nanoporous titanium surfaces for sustained elution of proteins and antibiotics. PLoS ONE 9(3):e92080. https://doi.org/10.1371/journal.pone.0092080
Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D (1998) Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res 40(1):1–11. https://doi.org/10.1002/(sici)1097-4636(199804)40:1%3c1:aid-jbm1%3e3.0.co;2-q
Cho S-A, Park K-T (2003) The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials 24(20):3611–3617. https://doi.org/10.1016/S0142-9612(03)00218-7
Kaczmarek D, Domaradzki J, Wojcieszak D, Prociow E, Mazur M, Placido F, Lapp S (2012) Hardness of nanocrystalline TiO2 thin films. J Nano Res 18–19:195–200. https://doi.org/10.4028/www.scientific.net/JNanoR.18-19.195
Hajizadeh K, Eghbali B, Topolski K, Kurzydlowski KJ (2014) Ultra-fine grained bulk CP-Ti processed by multi-pass ECAP at warm deformation region. Mater Chem Phys 143(3):1032–1038. https://doi.org/10.1016/j.matchemphys.2013.11.001
Zhang Y, Figueiredo RB, Alhajeri SN, Wang JT, Gao N, Langdon TG (2011) Structure and mechanical properties of commercial purity titanium processed by ECAP at room temperature. Mater Sci Eng A 528(25):7708–7714. https://doi.org/10.1016/j.msea.2011.06.054
Nishimoto S, Bhushan B (2013) Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv 3(3):671–690. https://doi.org/10.1039/C2RA21260A
Rupp F, Gittens RA, Scheideler L, Marmur A, Boyan BD, Schwartz Z, Geis-Gerstorfer J (2014) A review on the wettability of dental implant surfaces I: theoretical and experimental aspects. Acta Biomater 10(7):2894–2906. https://doi.org/10.1016/j.actbio.2014.02.040
Shibata Y, Tanimoto Y (2015) A review of improved fixation methods for dental implants. Part I: surface optimization for rapid osseointegration. J Prosthodont Res 59(1):20–33. https://doi.org/10.1016/j.jpor.2014.11.007
Hench L, Wilson J (1984) Surface-active biomaterials. Science 226(4675):630–636. https://doi.org/10.1126/science.6093253
Tong WP, Tao NR, Wang ZB, Lu J, Lu K (2003) Nitriding iron at lower temperatures. Science 299(5607):686–688. https://doi.org/10.1126/science.1080216
Yuan Y, Hays MP, Hardwidge PR, Kim J (2017) Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv 7(23):14254–14261. https://doi.org/10.1039/C7RA01571B
Skindersoe ME, Krogfelt KA, Blom A, Jiang G, Prestwich GD, Mansell JP (2015) Dual action of lysophosphatidate-functionalised titanium: interactions with human (MG63) osteoblasts and methicillin resistant Staphylococcus aureus. PLoS ONE 10(11):e0143509. https://doi.org/10.1371/journal.pone.0143509
Lüdecke C, Roth M, Yu W, Horn U, Bossert J, Jandt KD (2016) Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surf B 145:617–625. https://doi.org/10.1016/j.colsurfb.2016.05.049
Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L (2010) The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater 6(10):3824–3846. https://doi.org/10.1016/j.actbio.2010.04.001
Puckett SD, Taylor E, Raimondo T, Webster TJ (2010) The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 31(4):706–713. https://doi.org/10.1016/j.biomaterials.2009.09.081
Truong VK, Rundell S, Lapovok R, Estrin Y, Wang JY, Berndt CC, Barnes DG, Fluke CJ, Crawford RJ, Ivanova EP (2009) Effect of ultrafine-grained titanium surfaces on adhesion of bacteria. Appl Microbiol Biotechnol 83(5):925–937. https://doi.org/10.1007/s00253-009-1944-5
Mitik-Dineva N, Wang J, Truong VK, Stoddart P, Malherbe F, Crawford RJ, Ivanova EP (2009) Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol 58(3):268–273. https://doi.org/10.1007/s00284-008-9320-8
Mitik-Dineva N, Wang J, Mocanasu RC, Stoddart PR, Crawford RJ, Ivanova EP (2008) Impact of nano-topography on bacterial attachment. Biotechnol J 3(4):536–544. https://doi.org/10.1002/biot.200700244
Colon G, Ward BC, Webster TJ (2006) Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res Part A 78A(3):595–604. https://doi.org/10.1002/jbm.a.30789
Ploux L, Anselme K, Dirani A, Ponche A, Soppera O, Roucoules V (2009) Opposite responses of cells and bacteria to micro/nanopatterned surfaces prepared by pulsed plasma polymerization and UV-irradiation. Langmuir 25(14):8161–8169. https://doi.org/10.1021/la900457f
Preedy E, Perni S, Nipiĉ D, Bohinc K, Prokopovich P (2014) Surface roughness mediated adhesion forces between borosilicate glass and gram-positive bacteria. Langmuir 30(31):9466–9476. https://doi.org/10.1021/la501711t
Baek SM, Shin MH, Moon J, Jung HS, Lee SA, Hwang W, Yeom JT, Hahn SK, Kim HS (2017) Superior pre-osteoblast cell response of etched ultrafine-grained titanium with a controlled crystallographic orientation. Sci Rep 7:44213. https://doi.org/10.1038/srep44213
Vetrone F, Variola F, Tambasco de Oliveira P, Zalzal SF, Yi J-H, Sam J, Bombonato-Prado KF, Sarkissian A, Perepichka DF, Wuest JD, Rosei F, Nanci A (2009) Nanoscale oxidative patterning of metallic surfaces to modulate cell activity and fate. Nano Lett 9(2):659–665. https://doi.org/10.1021/nl803051f
Acknowledgements
The authors thank the Lloyd’s Register Foundation, UK (Project Number R265000553597 Nanotechnology in Sub-Sea Power Transmission) for research support. NKV acknowledges funding support from Lee Kong Chian School of Medicine, Nanyang Technological University Singapore Start-Up Grant (L0412290), and Strategic Academic Initiative Grant (SAI-L0494003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sandeep Kranthi Kiran, A., Sireesha, M., Ramalingam, R. et al. Modulation of biological properties by grain refinement and surface modification on titanium surfaces for implant-related infections. J Mater Sci 54, 13265–13282 (2019). https://doi.org/10.1007/s10853-019-03811-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03811-2