Abstract
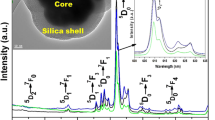
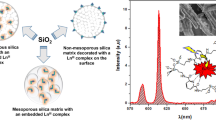
The work introduces silica confinement of Tb(III) and Yb(III) complexes with p-sulfonatothiacalix[4]arene (TCAS) arisen from their doping into silica nanoparticles (51–60 nm) as the reason for efficient dual green–NIR luminescence. pH-regulated water solubility of the lanthanide complexes is highlighted as prerequisite for the balance between efficient doping of the complexes into silica nanoparticles and their size/shape university. The impact of Tb(III) → Yb(III) energy transfer on the NIR and visible luminescence of the nanoparticles was revealed from photophysical studies of the nanoparticles doped with different couples of lanthanide complexes (Tb–Gd, Tb–Yb, Yb–Gd) at various molar ratios. The optimal balance between green and NIR luminescence is achieved for the silica nanoparticles doped with Tb–Yb complexes at 2:1 (Tb:Yb) ratio due to both Tb(III) → Yb(III) energy transfer and specific distribution of Tb(III) and Yb(III) complexes within silica nanoparticles. The dual luminescent nanoparticles exhibit efficient cellular uptake behavior after amino-decoration of their surface, which is confirmed by confocal microscopy images of the cells incubated by the heterometallic nanoparticles.






Similar content being viewed by others
References
Mao L, Liu Y, Yang S, Li Y, Zhang X, Wei Y (2019) Recent advances and progress of fluorescent bio-/chemosensors based on aggregation-induced emission molecules. Dyes Pigm 162:611–623
Zhang X, Wang K, Liu M, Zhang X, Tao L, Chen Y, Wei Y (2015) Polymeric AIE-based nanoprobes for biomedical applications: recent advances and perspectives. Nanoscale 7:11486–11508
Long Z, Mao L, Liu M, Wan Q, Wan Y, Zhang X, Wei Y (2017) Marrying multicomponent reactions and aggregation-induced emission (AIE): new directions for fluorescent nanoprobes. Polym Chem 8(37):5644–5654
Tian J, Jiang R, Gao P, Xu D, Mao L, Zeng G et al (2017) Synthesis and cell imaging applications of amphiphilic AIE-active poly(amino acid)s. Mater Sci Eng C Mater 79:563–569
Wan Q, Huang Q, Liu M, Xu D, Huang H, Zhang X, Wei Y (2017) Aggregation-induced emission active luminescent polymeric nanoparticles: non-covalent fabrication methodologies and biomedical applications. Appl Mater Today 9:145–160
Chen X, Zhou G, Peng X, Yoon J (2012) Biosensors and chemosensors based on the optical responses of polydiacetylenes. Chem Soc Rev 41(13):4610–4630
Stochaj U, Rodríguez Burbano DC, Cooper DR, Kodiha M, Capobianco JA (2018) The effects of lanthanide-doped upconverting nanoparticles on cancer cell biomarkers. Nanoscale 10:14464–14471
Wang J, Shah ZH, Zhang S, Lu R (2014) Silica-based nanocomposites via reverse microemulsions: classifications, preparations, and applications. Nanoscale 6:4418–4437
Makhinson B, Duncan AK, Elam AR, De Bettencourt-Dias A, Medley CD, Smith JE, Werner EJ (2013) Turning on lanthanide luminescence via nanoencapsulation. Inorg Chem 52:6311–6318
Nakahara Y, Tatsumi Y, Akimoto I, Osaki S, Doi M, Kimura K (2015) Fluorescent silica nanoparticles modified chemically with terbium complexes as potential bioimaging probes: their fluorescence and colloidal properties in water. New J Chem 39:1452–1458
Ibarra-Ruiz AM, Rodríguez Burbano DC, Capobianco JA (2016) Photoluminescent nanoplatforms in biomedical applications. Adv Phys X 1:194–225
Dong N-N, Pedroni M, Piccinelli F, Conti G, Sbarbati A, Ramírez-Hernández JE, Maestro LM, Iglesias-De La Cruz MC, Sanz-Rodriguez F, Juarranz A, Chen F, Vetrone F, Capobianco JA, Solé JG, Bettinelli M, Jaque D, Speghini A (2011) NIR-to-NIR two-photon excited CaF2:Tm3 + , Yb3 + nanoparticles: multifunctional nanoprobes for highly penetrating fluorescence bio-imaging. ACS Nano 5:8665–8671
Wang X, Liu K, Yang G, Cheng L, He L, Liu Y, Li Y, Guo L, Liu Z (2014) Near-infrared light triggered photodynamic therapy in combination with gene therapy using upconversion nanoparticles for effective cancer cell killing. Nanoscale 6:9198–9205
Yang G, Lv R, He F, Qu F, Gai S, Du S, Wei Z, Yang P (2015) A core/shell/satellite anticancer platform for 808 NIR light-driven multimodal imaging and combined chemo-/photothermal therapy. Nanoscale 7:13747–13758
Sun S-K, Wang H-F, Yan X-P (2018) Engineering persistent luminescence nanoparticles for biological applications: from biosensing/bioimaging to theranostics. Acc Chem Res 51:1131–1143
Du B, Cao X, Zhao F, Su X, Wang Y, Yan X, Jia S, Zhou J, Yao H (2016) Multimodal imaging-guided, dual-targeted photothermal therapy for cancer. J Mater Chem B 4:2038–2050
Li P, Yan Y, Chen B, Zhang P, Wang S, Zhou J, Fan H, Wang Y, Huang X (2018) Lanthanide-doped upconversion nanoparticles complexed with nano-oxide graphene used for upconversion fluorescence imaging and photothermal therapy. Biomater Sci 6:877–884
Bünzli J-CG (2015) On the design of highly luminescent lanthanide complexes. Coord Chem Rev 293–294:19–47
Bünzli J-CG (2016) Lanthanide light for biology and medical diagnosis. J Lumin 170:866–878
He F, Feng L, Yang P, Liu B, Gai S, Yang G, Dai Y, Lin J (2016) Enhanced up/down-conversion luminescence and heat: simultaneously achieving in one single core–shell structure for multimodal imaging guided therapy. Biomaterials 105:77–88
Mukhametshina AR, Mustafina AR, Davydov NA, Fedorenko SV, Nizameev IR, Kadirov MK, Gorbatchuk VV, Konovalov AI (2015) Tb(III)-doped silica nanoparticles for sensing: effect of interfacial interactions on substrate-induced luminescent response. Langmuir 31:611–619
Fedorenko SV, Mustafina AR, Mukhametshina AR, Jilkin ME, Mukhametzyanov TA, Solovieva AO, Pozmogova TN, Shestopalova LV, Shestopalov MA, Kholin KV, Osin YN, Sinyashin OG (2017) Cellular imaging by green luminescence of Tb(III)-doped aminomodified silica nanoparticles. Mater Sci Eng, C 76:551–558
Mukhametshina AR, Fedorenko SV, Zueva IV, Petrov KA, Masson P, Nizameev IR, Mustafina AR, Sinyashin OG (2016) Luminescent silica nanoparticles for sensing acetylcholinesterase-catalyzed hydrolysis of acetylcholine. Biosens Bioelectron 77:871–878
Mukhametshina AR, Fedorenko SV, Petrov AM, Zakyrjanova GF, Petrov KA, Nurullin LF, Nizameev IR, Mustafina AR, Sinyashin OG (2018) Targeted nanoparticles for selective marking of neuromuscular junctions and ex vivo monitoring of endogenous acetylcholine hydrolysis. ACS Appl Mater Interfaces 10:14948–14955
Mukhametshina A, Petrov A, Fedorenko S, Petrov K, Nizameev I, Mustafina A, Sinyashin O (2018) Luminescent nanoparticles for rapid monitoring of endogenous acetylcholine release in mice atria. Luminescence 33:588–593
Comby S, Surender EM, Kotova O, Truman LK, Molloy JK, Gunnlaugsson T (2014) Lanthanide-functionalized nanoparticles as MRI and luminescent probes for sensing and/or imaging applications. Inorg Chem 53:1867–1879
Liu Q, Zou X, Shi Y, Shen B, Cao C, Cheng S, Feng W, Li F (2018) An efficient dye-sensitized NIR emissive lanthanide nanomaterial and its application in fluorescence-guided peritumoral lymph node dissection. Nanoscale 10:12573–12581
Zatsepin A, Kuznetsova Y (2018) Down-conversion of UV radiation in erbium-doped gadolinium oxide nanoparticles. Appl Mater Today 12:34–42
Chen D-M, Sun C-X, Peng Y, Zhang N-N, Si H-H, Liu C-S, Du M (2018) Ratiometric fluorescence sensing and colorimetric decoding methanol by a bimetallic lanthanide-organic framework. Sens Actuators, B 265:104–109
Mustafina AR, Fedorenko SV, Konovalova OD, Menshikova AY, Shevchenko NN, Soloveva SE, Konovalov AI, Antipin IS (2009) Novel highly charged silica-coated Tb(III) nanoparticles with fluorescent properties sensitive to ion exchange and energy transfer processes in aqueous dispersions. Langmuir 25(5):3146–3151. https://doi.org/10.1021/la8032572
Fedorenko SV, Bochkova OD, Mustafina AR, Burilov VA, Kadirov MK, Holin CV, Nizameev IR, Skripacheva VV, Menshikova AY, Antipin IS, Konovalov AI (2010) Dual visible and near-infrared luminescent silica nanoparticles. Synthesis and aggregation stability. J Phys Chem C 114:6350–6355
Iki N, Fujimoto T, Miyano S (1998) A new water-soluble host molecule derived from thiacalixarene. Chem Lett 27:625–626
Davydov N, Mustafina A, Burilov V, Zvereva E, Katsyuba S, Vagapova L, Konovalov A, Antipin I (2012) Complex formation of d-metal ions at the interface of TbIII-doped silica nanoparticles as a basis of substrate-responsive TbIII-centered luminescence. ChemPhysChem 13:3357–3364
Chen Y, Zhang Y (2011) Fluorescent quantification of amino groups on silica nanoparticle surfaces. Anal Bioanal Chem 399:2503–2509
Voloshina AD, Semenov VE, Strobykina AS, Kulik NV, Krylova ES, Zobov VV, Reznik VS (2017) Synthesis and antimicrobial and toxic properties of novel 1,3-bis(alkyl)-6-methyluracil derivatives containing 1,2,3- and 1,2,4-triazolium fragments. Russ J Bioorg Chem 43(2):170–176
Arriagada FJ, Osseo-Asare K (1999) Controlled hydrolysis of tetraethoxysilane in a nonionic water-in-oil microemulsion: a statistical model of silica nucleation. Colloids Surf A 154(3):311–326
Bagwe RP, Yang C, Hilliard LR, Tan W (2004) Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir 20(19):8336–8342
Fedorenko SV, Grechkina SL, Mustafina AR, Kholin KV, Stepanov AS, Nizameev IR, Ismaev IE, Kadirov MK, Zairov RR, Fattakhova AN, Amirov RR, Soloveva SE (2017) Tuning the non-covalent confinement of Gd(III) complexes in silica nanoparticles for high T1-weighted MR imaging capability. Colloids Surf B 149:243–249
Karashimada R, Iki N (2016) Thiacalixarene assembled heterotrinuclear lanthanide clusters comprising TbIII and YbIII enable f–f communication to enhance YbIII-centred luminescence. Chem Commun 52:3139–3142
Iki N, Hiro-oka S, Nakamura M, Tanaka T, Hoshino H (2012) Kinetically stable LnIII complexes comprising a trinuclear core sandwiched between two thiacalix[4]arene ligands self-assembled in water (LnIII = NdIII, YbIII). Eur J Inorg Chem 2012:3541–3545
Amirov R, McMillan Z, Mustafina A, Chukurova I, Solovieva S, Antipin I, Konovalov A (2005) A first report on ternary complex formation between p-sulfonatothiacalix[4]arene, tetramethylammonium ion and gadolinium (III) ion in aqueous solutions. Inorg Chem Commun 8(9):821–824
Bonacchi S, Genovese D, Juris R, Marzocchi E, Montalti M, Prodi L, Rampazzo E, Zaccheroni N (2008) In: Geddes CD (ed) Reviews in fluorescence. Springer, Baltimore, pp 119–137
Zhang Y, Lv J, Ding N, Jiang S, Zheng T, Li J (2015) Tunable luminescence and energy transfer from Gd3+ to Tb3+ ions in silicate oxyfluoride scintillating glasses via varying Tb3+ concentration. J Non-Cryst Solids 423–424:30–34
Zairov R, Khakimullina G, Podyachev S, Nizameev I, Safiullin G, Amirov R, Vomiero A, Mustafina A (2017) Hydration number: crucial role in nuclear magnetic relaxivity of Gd(III) chelate-based nanoparticles. Sci Rep. https://doi.org/10.1038/s41598-017-14409-6
Larson DR, Ow H, Vishwasrao HD, Heikal AA, Wiesner U, Webb WW (2008) Silica nanoparticle architecture determines radiative properties of encapsulated fluorophores. Chem Mater 20:2677–2684
Iki N, Ohta M, Tanaka T, Horiuchi T, Hoshino H (2009) A supramolecular sensing system for AgI at nanomolar levels by the formation of a luminescent AgI–TbIII–thiacalix[4]arene ternary complex. New J Chem 33(1):23–25
Saha K, Kim ST, Yan B, Miranda OR, Alfonso FS, Shlosman D, Rotello VM (2013) Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small 9:300–305
Shahabi S, Treccani L, Dringen R, Rezwan K (2015) Modulation of silica nanoparticle uptake into human osteoblast cells by variation of the ratio of amino and sulfonate surface groups: effects of serum. ACS Appl Mater Interfaces 7:13821–13833
Guarnieri D, Malvindi MA, Belli V, Pompa PP, Netti P (2014) Effect of silica nanoparticles with variable size and surface functionalization on human endothelial cell viability and angiogenic activity. J Nanopart Res. https://doi.org/10.1007/s11051-013-2229-6
Zhu J, Liao L, Zhu L, Zhang P, Guo K, Kong J, Ji C, Liu B (2013) Size-dependent cellular uptake efficiency, mechanism, and cytotoxicity of silica nanoparticles toward HeLa cells. Talanta 107:408–415
Colthup NB, Daly LH, Wiberley SE (1964) Introduction to infrared and Raman spectroscopy. Academic Press, New York, p 278
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Kipen HM, Laskin DL (2005) Smaller is not always better: nanotechnology yields nanotoxicology. Am J Physiol Lung Cell Mol Physiol 289:L696–L697
Acknowledgements
The authors are grateful to the Assigned Spectral-Analytical Center of FRC Kazan Scientific Center of RAS for technical assistance in research. The authors are grateful to Interdisciplinary Center for Analytical Microscopy at KFU for assistance with the confocal microscopy experiments. Authors thank Dr I.I. Vandykova for help in IR measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fedorenko, S., Gilmanova, D., Mukhametshina, A. et al. Silica nanoparticles with dual visible–NIR luminescence affected by silica confinement of Tb(III) and Yb(III) complexes for cellular imaging application. J Mater Sci 54, 9140–9154 (2019). https://doi.org/10.1007/s10853-019-03532-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03532-6