Abstract
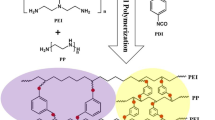
Acid-resistant membrane has an important application prospect in the field of industrial wastewater treatment. Polysulfonamide (PSA) filtration membrane possessing good stability in acidic condition was often hampered for application due to the poor performance by the state-of-the-art interfacial polymerization (IP) preparation method. Herein, a spinning-assist multilayer interfacial polymerization (sMIP) method was devised and employed for the fabrication of a PSA thin-film composite (TFC) membrane from piperazine (PIP) and 2,4,6-tris(chlorosulfonyl) phenol (TCSP). Membrane fabricated with 5 layers by sMIP method exhibited greater than 98% rejection rate for Na2SO4 and MgSO4 and outperformed the control IP group by ~ 147% enhanced water permeance and a magnitude greater permselectivity due to a reduced active layer thickness. Compared with polyamide membranes, the PSA TFC membrane exhibited better stability toward acid through a high-temperature treatment in a 20% H2SO4 aqueous solution. For a CuSO4 and H2SO4 mixed solution filtration, the PSA membrane exhibited good permselectivity with CuSO4 rejection of 78% and H2SO4 rejection of 8% at a permeate flux of 13.98 L m−2 h−1. These results have demonstrated that the sMIP method provides an effective way to fabricate polysulfonamide membrane with excellent salt rejection as well as appreciable water permeance.













Similar content being viewed by others
References
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P (2009) Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res 43(9):2317–2348. https://doi.org/10.1016/j.watres.2009.03.010
Van der Bruggen B, Vandecasteele C (2003) Removal of pollutants from surface water and groundwater by nanofiltration: overview of possible applications in the drinking water industry. Environ Pollut 122(3):435–445. https://doi.org/10.1016/S0269-7491(02)00308-1
Werber JR, Osuji CO, Elimelech M (2016) Materials for next-generation desalination and water purification membranes. Nat Rev Mater 1(5):16018. https://doi.org/10.1038/natrevmats.2016.18
Platt S, Nyström M, Bottino A, Capannelli G (2004) Stability of NF membranes under extreme acidic conditions. J Membr Sci 239(1):91–103. https://doi.org/10.1016/j.memsci.2003.09.030
Agboola O, Schoeman JJ, Maree J, Mbaya R, Kolesnikov A (2012) Performance of an acid stable nanofiltration membrane for nickel removal from aqueous solutions: effects of concentration, solution pH and ionic strength. WIT Trans Ecol Environ 163:415–424. https://doi.org/10.2495/WM120371
Gomes S, Cavaco SA, Quina MJ, Gando-Ferreira LM (2010) Nanofiltration process for separating Cr(III) from acid solutions: experimental and modelling analysis. Desalination 254(1–3):80–89. https://doi.org/10.1016/j.desal.2009.12.010
Tanninen J, Mänttäri M, Nyström M (2006) Nanofiltration of concentrated acidic copper sulphate solutions. Desalination 189(1–3):92–96. https://doi.org/10.1016/j.desal.2005.06.017
Tanninen J (2004) Long-term acid resistance and selectivity of NF membranes in very acidic conditions. J Membr Sci 240(1–2):11–18. https://doi.org/10.1016/j.memsci.2004.04.006
Ricci BC, Ferreira CD, Marques LS, Martins SS, Reis BG, Amaral MCS (2017) Assessment of the chemical stability of nanofiltration and reverse osmosis membranes employed in treatment of acid gold mining effluent. Sep Purif Technol 174:301–311. https://doi.org/10.1016/j.seppur.2016.11.007
Visser TJK, Modise SJ, Krieg HM, Keizer K (2001) The removal of acid sulphate pollution by nanofiltration. Desalination 140(1):79–86. https://doi.org/10.1016/S0011-9164(01)00356-3
Zhang J, Yue L, Kong Q, Liu Z, Zhou X, Zhang C, Pang S, Wang X, Yao J, Cui G (2013) A heat-resistant silica nanoparticle enhanced polysulfonamide nonwoven separator for high-performance lithium ion battery. J Electrochem Soc 160(6):A769–A774. https://doi.org/10.1149/2.043306jes
Zhang J, Wen H, Yue L, Chai J, Ma J, Hu P, Ding G, Wang Q, Liu Z, Cui G, Chen L (2017) In situ formation of polysulfonamide supported poly(ethylene glycol) divinyl ether based polymer electrolyte toward monolithic sodium ion batteries. Small 13(2):1601530. https://doi.org/10.1002/smll.201601530
Baxter NJ, Rigoreau LJM, Laws AP, Page MI (2000) Reactivity and mechanism in the hydrolysis of β-Sultams. J Am Chem Soc 122(14):3375–3385. https://doi.org/10.1021/ja994293b
Searles S, Nukina S (1959) Cleavage And Rearrangement Of Sulfonamides. Chem Rev 59(6):1077–1103. https://doi.org/10.1021/cr50030a004
Kurth CJ, Kloos SD, Peschl JA, Hodgins LT (2001) US7138058, Acid stable membranes for nanofiltration
Liu M, Yao G, Cheng Q, Ma M, Yu S, Gao C (2012) Acid stable thin-film composite membrane for nanofiltration prepared from naphthalene-1,3,6-trisulfonylchloride (NTSC) and piperazine (PIP). J Membr Sci 415–416:122–131. https://doi.org/10.1016/j.memsci.2012.04.043
Hoseinpour H, Peyravi M, Nozad A, Jahanshahi M (2016) Static and dynamic assessments of polysulfonamide and poly(amide-sulfonamide) acid-stable membranes. J Taiwan Inst Chem Eng 67:453–466. https://doi.org/10.1016/j.jtice.2016.07.039
Chai G-Y, Krantz WB (1994) Formation and characterization of polyamide membranes via interfacial polymerization. J Membr Sci 93(2):175–192. https://doi.org/10.1016/0376-7388(94)80006-5
Karan S, Jiang Z, Livingston AG (2015) Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348(6241):1347–1351. https://doi.org/10.1126/science.aaa5058
Jimenez-Solomon MF, Song Q, Jelfs KE, Munoz-Ibanez M, Livingston AG (2016) Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat Mater 15(7):760–767. https://doi.org/10.1038/nmat4638
Cadotte J E (1981) US4277344. Interfacially synthesized reverse osmosis membrane
Miller DJ, Dreyer DR, Bielawski CW, Paul DR, Freeman BD (2017) Surface modification of water purification membranes. Angew Chem Int Ed Engl 56(17):4662–4711. https://doi.org/10.1002/anie.201601509
Paul M, Jons SD (2016) Chemistry and fabrication of polymeric nanofiltration membranes: a review. Polymer 103:417–456. https://doi.org/10.1016/j.polymer.2016.07.085
Park HB, Kamcev J, Robeson LM, Elimelech M, Freeman BD (2017) Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356(6343):eaab0530. https://doi.org/10.1126/science.aab0530
Putkonen M, Harjuoja J, Sajavaara T, Niinisto L (2007) Atomic layer deposition of polyimide thin films. J Mater Chem 17(7):664–669. https://doi.org/10.1039/B612823H
Choi W, Gu J-E, Park S-H, Kim S, Bang J, Baek K-Y, Park B, Lee JS, Chan EP, Lee J-H (2015) Tailor-made polyamide membranes for water desalination. ACS Nano 9(1):345–355. https://doi.org/10.1021/nn505318v
Gu JE, Lee S, Stafford CM, Lee JS, Choi W, Kim BY, Baek KY, Chan EP, Chung JY, Bang J, Lee JH (2013) Molecular layer-by-layer assembled thin-film composite membranes for water desalination. Adv Mater 25(34):4778–4782. https://doi.org/10.1002/adma.201302030
Johnson PM, Yoon J, Kelly JY, Howarter JA, Stafford CM (2012) Molecular layer-by-layer deposition of highly crosslinked polyamide films. J Polym Sci Part B Polym Phys 50(3):168–173. https://doi.org/10.1002/polb.23002
Choi W, Jeon S, Kwon SJ, Park H, Park Y-I, Nam S-E, Lee PS, Lee JS, Choi J, Hong S, Chan EP, Lee J-H (2017) Thin film composite reverse osmosis membranes prepared via layered interfacial polymerization. J Membr Sci 527:121–128. https://doi.org/10.1016/j.memsci.2016.12.066
Song X, Qi S, Tang CY, Gao C (2017) Ultra-thin, multi-layered polyamide membranes: synthesis and characterization. J Membr Sci 540:10–18. https://doi.org/10.1016/j.memsci.2017.06.016
Freger V (2005) Kinetics of film formation by interfacial polycondensation. Langmuir 21(5):1884–1894. https://doi.org/10.1021/la048085v
Wadekar SS, Vidic RD (2017) Influence of active layer on separation potentials of nanofiltration membranes for inorganic ions. Environ Sci Technol 51(10):5658–5665. https://doi.org/10.1021/acs.est.6b05973
Boiko VN, Filatov AA, Yagupolskii YL, Tyrra W, Naumann D, Pantenburg I, Fischer HTM, Schulz F (2011) A convenient synthetic route to 2,4,6-tris(chlorosulfonyl)- and 2,4,6-tris(fluorosulfonyl)phenol, aniline and chlorobenzene. J Fluor Chem 132(12):1219–1226. https://doi.org/10.1016/j.jfluchem.2011.06.045
Freger V, Bottino A, Capannelli G, Perry M, Gitis V, Belfer S (2005) Characterization of novel acid-stable NF membranes before and after exposure to acid using ATR-FTIR, TEM and AFM. J Membr Sci 256:134–142. https://doi.org/10.1016/j.memsci.2005.02.014
Siow KS, Britcher L, Kumar S, Griesser HJ (2009) Sulfonated surfaces by sulfur dioxide plasma surface treatment of plasma polymer films. Plasma Processes Polym 6(9):583–592. https://doi.org/10.1002/ppap.200950004
Kim SH, Kwak S-Y, Suzuki T (2005) Positron annihilation spectroscopic evidence to demonstrate the flux-enhancement mechanism in morphology-controlled thin-film-composite (TFC) membrane. Environ Sci Technol 39(6):1764–1770. https://doi.org/10.1021/es049453k
Geise GM, Paul DR, Freeman BD (2014) Fundamental water and salt transport properties of polymeric materials. Prog Polym Sci 39(1):1–42. https://doi.org/10.1016/j.progpolymsci.2013.07.001
Geise GM, Park HB, Sagle AC, Freeman BD, McGrath JE (2011) Water permeability and water/salt selectivity tradeoff in polymers for desalination. J Membr Sci 369(1–2):130–138. https://doi.org/10.1016/j.memsci.2010.11.054
Peeters JMM, Boom JP, Mulder MHV, Strathmann H (1998) Retention measurements of nanofiltration membranes with electrolyte solutions. J Membr Sci 145(2):199–209. https://doi.org/10.1016/S0376-7388(98)00079-9
Schäfer AI, Fane AG, Waite TD (2005) Nanofiltration: principles and applications. Elsevier, Amsterdam
Sun J, Zhang L, Xie B, Fan L, Yu S (2013) Separation efficiency and stability of thin-film composite nanofiltration membranes in long-term filtration of copper sulphate and sulphuric acid mixture. Desalination Water Treatment 53(7):1822–1833. https://doi.org/10.1080/19443994.2013.860629
Aharoni SM (1992) The solubility parameters of aromatic polyamides. J Appl Polym Sci 45(5):813–817. https://doi.org/10.1002/app.1992.070450507
Tang CY, Kwon Y-N, Leckie JO (2009) Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes. Desalination 242(1):149–167. https://doi.org/10.1016/j.desal.2008.04.003
Acknowledgements
The authors gratefully acknowledge the financial support from the Fundamental Research Funds for the Central Universities (No. 15CX02015A, 16CX05009A, 18CX05006A), the National Natural Science Foundation of China (Grant No. 21502227), the Province Key Research and Development Program of Shandong (No. 2016GSF115032), Postdoctoral application Program of Qingdao (No. T1604013), the State Key Laboratory of Separation Membranes and Membrane Processes (Tianjin Polytechnic University, No. M1-201601), and State Key Laboratory of Heavy Oil Processing SLKZZ-2017009.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
He, M., Yuan, T., Dong, W. et al. High-performance acid-stable polysulfonamide thin-film composite membrane prepared via spinning-assist multilayer interfacial polymerization. J Mater Sci 54, 886–900 (2019). https://doi.org/10.1007/s10853-018-2847-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2847-6