Abstract
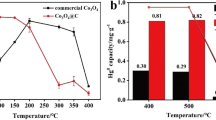
Herein, we reported a facile method for fabricating nanoflower-like Co3O4 catalysts via calcination treatment based on ZIF-67. The catalytic performances of the obtained Co3O4 catalysts were evaluated for the model reaction of CO oxidation. The results demonstrated that calcination temperature had a strong effect on the structure and catalytic reaction activity of Co3O4 catalyst. Co3O4 catalyst prepared at 400 °C (Co3O4-400) exhibited the optimum catalytic activity with a complete CO conversion temperature of 105 °C. This phenomenon was ascribed to the higher specific surface areas, smaller particle size, unique structure, good low-temperature reduction and higher abundances of surface Co2+ and adsorbed oxygen species. The addition of 1.0% water vapor had a negative effect on CO oxidation and the prepared Co3O4-400 catalyst presented long-term stability.











Similar content being viewed by others
References
Yang YQ, Dong H, Wang Y, Wang YX, Liu N, Wang DJ, Zhang XD (2017) A facile synthesis for porous CuO/Cu2O composites derived from MOFs and their superior catalytic performance for CO oxidation. Inorg Chem Commun 86:74–77. https://doi.org/10.1016/j.inoche.2017.09.027
Yang YQ, Dong H, Wang Y, He C, Wang YX, Zhang XD (2018) Synthesis of octahedral like Cu-BTC derivatives derived from MOF calcined under different atmosphere for application in CO oxidation. J Solid State Chem 258:582–587. https://doi.org/10.1016/j.jssc.2017.11.033
Cui LF, Zhao D, Yang Y, Wang YX, Zhang XD (2017) Synthesis of highly efficient α-Fe2O3 catalysts for CO oxidation derived from MIL-100(Fe). J Solid State Chem 247:168–172. https://doi.org/10.1016/j.jssc.2017.01.013
Zhang XD, Dong H, Wang Y, Liu N, Zuo YH, Cui LF (2016) Study of catalytic activity at the Ag/Al-SBA-15 catalysts for CO oxidation and selective CO oxidation. Chem Eng J 283:1097–1107. https://doi.org/10.1016/j.cej.2015.08.064
Zheng FC, Yin ZC, Xu SH, Zhang YG (2016) Formation of Co3O4 hollow polyhedrons from metal-organic frameworks and their catalytic activity for CO oxidation. Mater Lett 182:214–217. https://doi.org/10.1016/j.matlet.2016.06.108
Lv S, Xia G, Jin C, Hao C, Wang L, Li J, Zhang Y, Zhu JJ (2016) Low-temperature CO oxidation by Co3O4 nanocubes on the surface of Co(OH)2 nanosheets. Catal Commun 86:100–103. https://doi.org/10.1016/j.catcom.2016.08.020
Xie XW, Li Y, Liu ZQ, Haruta M, Shen W (2009) Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458(7239):746–749. https://doi.org/10.1038/nature07877
Yu FL, Qu ZP, Zhang XD, Fu Q, Wang Y (2013) Investigation of CO and formaldehyde oxidation over mesoporous Ag/Co3O4 catalysts. J Energy Chem 22:845–852. https://doi.org/10.1016/S2095-4956(14)60263-1
Hu LH, Sun KQ, Peng Q, Xu BQ, Li YD (2010) Surface active sites on Co3O4 nanobelt and nanocube model catalysts for CO oxidation. Nano Res 3:363–368. https://doi.org/10.1007/s12274-010-1040-2
Song W, Poyraz AS, Meng Y, Ren Z, Chen SY, Suib SL (2014) Mesoporous Co3O4 with controlled porosity: inverse micelle synthesis and high-performance catalytic CO oxidation at − 60 °C. Chem Mater 26:4629–4639. https://doi.org/10.1021/cm502106v
Huang WY, Liu N, Zhang XD, Wu MH, Tang L (2017) Metal organic framework g-C3N4/MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Appl Surf Sci 425:107–116. https://doi.org/10.1016/j.apsusc.2017.07.050
Liu N, Huang WY, Zhang XD, Tang L, Wang L, Wang YX, Wu MH (2018) Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB. Appl Catal B 221:119–128. https://doi.org/10.1016/j.apcatb.2017.09.020
Zhang XD, Yang Y, Huang WY, Yang YQ, Wang YX, He C, Liu N, Wu MH, Tang L (2018) g-C3N4/UiO-66 nanohybrids with enhanced photocatalytic activities for the oxidation of dye under visible light irradiation. Mater Res Bull 99:349–358. https://doi.org/10.1016/j.materresbull.2017.11.028
Zhang XD, Li HX, Lv XT, Xu JC, Wang YX, He C, Liu N, Yang YQ, Wang Y (2018) Facile synthesis of highly efficient amorphous Mn-MIL-100 catalysts: the formation mechanism and the structure changes during the application for CO oxidation. Chem Eur J 24:8822–8832. https://doi.org/10.1002/chem.201800773
Zhang XD, Hou FL, Yang Y, Wang Y, Liu N, Chen D, Yang YQ (2017) A facile synthesis for cauliflower like CeO2 catalysts from Ce-BTC precursor and their catalytic performance for CO oxidation. Appl Surf Sci 423:771–779. https://doi.org/10.1016/j.apsusc.2017.06.235
Zhang XD, Yang Y, Song L, Wang YX, He C, Wang Z, Cui LF (2018) High and stable catalytic activity of Ag/Fe2O3 catalysts derived from MOFs for CO oxidation. Mol Catal 447:80–89. https://doi.org/10.1016/j.mcat.2018.01.007
Zhang XD, Yang Y, Lv XT, Wang YX, Cui LF (2017) Effects of preparation method on the structure and catalytic activity of Ag-Fe2O3 catalysts derived from MOFs. Catalysts 7:382. https://doi.org/10.3390/catal7120382
Borhani S, Moradia M, Kiani MA, Hajati S, Toth J (2017) CoxZn1−x ZIF-derived binary Co3O4/ZnO wrapped by 3D reduced graphene oxide for asymmetric supercapacitor: comparison of pure and heat-treated bimetallic MOF. Ceram Int 43:14413–14425. https://doi.org/10.1016/j.ceramint.2017.07.211
Bigdeli H, Moradi M, Hajati S, Kiani MA, Toth J (2017) Cobalt terephthalate MOF-templated synthesis of porous nano-crystalline Co3O4 by the new indirect solid state thermolysis as cathode material of asymmetric supercapacitor. Physica E 94:158–166. https://doi.org/10.1016/j.physe.2017.08.005
Wang WX, Li YW, Zhang RJ, He DH, Liu HL, Liao SJ (2011) Metal-organic framework as a host for synthesis of nanoscale Co3O4 as an active catalyst for CO oxidation. Catal Commun 12:875–879. https://doi.org/10.1016/j.catcom.2011.02.001
Yan N, Chen QW, Wang F, Wang Y, Zhong H, Hu L (2012) High catalytic activity for CO oxidation of Co3O4 nanoparticles in SiO2 nanocapsules. J Mater Chem A 1:637–643. https://doi.org/10.1039/C2TA00132B
Bao SX, Yan N, Shi XH, Li R, Chen QW (2014) High and stable catalytic activity of porous Ag/Co3O4 nanocomposites derived from MOFs for CO oxidation. Appl Catal A 487:189–194. https://doi.org/10.1016/j.apcata.2014.09.015
Zheng FC, Yin ZC, Xu SH, Zhang YG (2016) Formation of Co3O4 hollow polyhedrons from metal-organic frameworks and their catalytic activity for CO oxidation. Mater Lett 182:214–217. https://doi.org/10.1016/j.matlet.2016.06.108
Zhang C, Zhang L, Xu GC, Ma X, Li YH, Zhang CY, Jia DZ (2017) Metal organic framework-derived Co3O4 microcubes and their catalytic applications in CO oxidation. New J Chem 41:1631–1636. https://doi.org/10.1039/c6nj02507b
Li GQ, Zhang CH, Wang Z, Huang H, Peng H, Li XB (2018) Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl Catal A 550:67–76. https://doi.org/10.1016/j.apcata.2017.11.003
Shen LS, Wang CX (2014) Hierarchical Co3O4 nanoparticles embedded in a carbon matrix for lithium-ion battery anode materials. Electrochim Acta 133:16–22. https://doi.org/10.1016/j.electacta.2014.03.182
Li GC, Hua XN, Liu PF, Xie YX, Han L (2015) Porous Co3O4 microflowers prepared by thermolysis of metal-organic framework for supercapacitor. Mater Chem Phys 168:127–131. https://doi.org/10.1016/j.matchemphys.2015.11.011
Shahabuddin S, Muhamad Sarih N, Mohamad S, Baharin SNA (2016) Synthesis and characterization of Co3O4 nanocube doped polyaniline nanocomposites with enhanced methyl orange adsorption from aqueous solution. RSC Adv 6(49):43388–43400. https://doi.org/10.1039/C6RA04757B
Shahida MM, Rameshkumar P, Basirunc WJ, Chingd JC, Huange NM (2017) Cobalt oxide nanocubes interleaved reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction in alkaline medium. Electrochim Acta 237:61–68. https://doi.org/10.1016/j.electacta.2017.03.088
Kleitz F, Berube F, Guillet-Nicolas R, Yang CM, Thommes M (2010) Probing adsorption, pore condensation, and hysteresis behavior of pure fluids in three-dimensional cubic mesoporous KIT-6 silica. J Phys Chem C 114:9344–9355. https://doi.org/10.1021/jp909836v
Ma CY, Wang DH, Xue WJ, Dou BJ, Wang HL, Hao ZP (2011) Investigation of formaldehyde oxidation over Co3O4-Ce2 and Au/Co3O4-CeO2 catalysts at room temperature: effective removal and determination of reaction mechanism. Environ Sci Technol 45:3628–3634. https://doi.org/10.1021/es104146v
Ma CY, Mu Z, Li JJ, Jin YG, Cheng J, Lu GQ, Hao ZP, Qiao SZ (2010) Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J Am Chem Soc 132:2608–2613. https://doi.org/10.1021/ja906274t
Zhang YJ, Zhang L, Deng JG, Xie SH, Yang HG, Jiang Y, Dai HX (2015) Synthesis, characterization, and catalytic properties of MnOx/SBA-16 for toluene oxidation. In: Proceedings of the 2014 international conference on materials science and energy engineering (Cmsee 2014), p 154. https://doi.org/10.1142/9789814678971_0024
Zhang XD, Li HX, Hou FL, Yang Y, Dong H, Liu N, Wang YX, Cui LF (2017) Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. Appl Surf Sci 411:27–33. https://doi.org/10.1016/j.apsusc.2017.03.179
Tang CW, Yu WY, Lin CJ, Wang CB, Chien SH (2007) Phase transformation in CeO2-Co3O4 binary oxide under reduction and calcination pretreatments. Catal Lett 116:161–166. https://doi.org/10.1007/s10562-007-9105-x
Baidya T, Murayama T, Bera P, Safonova OV, Steiger P, Katiyar NK, Biswas K, Haruta M (2017) Low-temperature CO oxidation over combustion made Fe- and Cr-doped Co3O4 catalysts: role of dopant’s nature toward achieving superior catalytic activity and stability. J Phys Chem C 121:15256–15265. https://doi.org/10.1021/acs.jpcc.7b04348
Yan XD, Tian LH, He M, Chen XB (2015) Three-dimensional crystalline/amorphous Co/Co3O4 core/shell nanosheets as efficient electrocatalysts for the hydrogen evolution reaction. Nano Lett 15:6015–6021. https://doi.org/10.1021/acs.nanolett.5b02205
Cai T, Huang H, Deng W, Dai QG, Liu W, Wang XY (2015) Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl Catal B 166:393–405. https://doi.org/10.1016/j.apcatb.2014.10.047
Rokicińska A, Natkański P, Dudek B, Drozdek M, Lityńska-Dobrzyńska L, Kuśtrowski P (2016) Co3O4-pillared montmorillonite catalysts synthesized by hydrogel-assisted route for total oxidation of toluene. Appl Catal B 195:59–68. https://doi.org/10.1016/j.apcatb.2016.05.008
Jiang Y, Xie SH, Yang HG, Deng JG, Liu YX, Dai HX (2017) Mn3O4-Au/3DOM La0.6Sr0.4CoO3: high-performance catalysts for toluene oxidation. Catal Today 281:437–446. https://doi.org/10.1016/j.apcatb.2016.05.008
Wang FG, Zhang LJ, Xu LL, Deng ZY, Shi WD (2017) Low temperature CO oxidation and CH4 combustion over Co3O4 nanosheets. Fuel 203:419–429. https://doi.org/10.1016/j.fuel.2017.04.140
Ding K, Wang D, Yang P, Hou PK, Cheng X (2016) Enhanced CO catalytic oxidation of flower-like Co3O4 composed of small nanoparticles. RSV Adv 6:16208–16214. https://doi.org/10.1039/C6RA01092J
Wang J, Zhong LP, Lu JC, Chen R, Lei YQ, Chen KZ, Han CY, He SF, Wan GP, Luo YM (2017) A solvent-free method to rapidly synthesize CuO-CeO2 catalysts to enhance their CO preferential oxidation: effects of Cu loading and calcination temperature. Mol Catal 443:241–252. https://doi.org/10.1016/j.mcat.2017.10.012
Wu MZ, Zhan WC, Guo Y, Wang YS, Guo YL, Gong XQ, Wang L, Lu GZ (2016) Solvent free selective oxidation of cyclohexane with molecular oxygen over manganese oxides: effect of the calcination temperature. Chin J Catal 37:184–192. https://doi.org/10.1016/S1872-2067(15)60983-4
Liu CX, Liu Q, Bai L, Dong AQ, Liu GB, Wen SH (2013) Structure and catalytic performances of nanocrystalline Co3O4 catalysts for low temperature CO oxidation prepared by dry and wet synthetic routes. J Mol Catal A 370:1–6. https://doi.org/10.1016/j.molcata.2012.12.003
Wang C, Tian CC, Guo YL, Zhang ZD, Hua WC, Zhan WC, Guo Y, Wang L, Lu GZ (2018) Ruthenium oxides supported on heterostructured CoPO-MCF materials for catalytic oxidation of vinyl chloride emissions. J Hazard Mater 342:290–296. https://doi.org/10.1016/j.jhazmat.2017.08.036
Rousseau S, Loridant S, Delichere P, Boreave A, Deloume JP, Vernoux P (2009) La(1-x)SrxCo1-yFeyO3 perovskites prepared by sol-gel method: characterization and relationships with catalytic properties for total oxidation of toluene. Appl Catal B 88:438–447. https://doi.org/10.1016/j.apcatb.2008.10.022
Prasad R, Singh P (2012) A review on CO oxidation over copper chromite catalyst. Catal Rev 54:224. https://doi.org/10.1080/01614940.2012.648494
El Kasmi A, Tian ZY, Vieker H, Beyer A, Chafik T (2016) Innovative CVD synthesis of Cu2O catalysts for CO oxidation. Appl Catal B 186:10–18. https://doi.org/10.1016/j.apcatb.2015.12.034
Naofumi U, Masayuki U, Jun WY, Kazuyuki K (2002) Synthesis of CeO2 spherical fine particles by homogeneous precipitation method with polyethylene glycol. Chem Lett 31:854–855. https://doi.org/10.1246/cl.2002.854
Gao Y, Shao N, Pei Y, Chen ZF, Zeng XC (2011) Catalytic activities of subnanometer gold clusters (Au16-Au18, Au20, and Au27-Au35) for CO oxidation. ACS Nano 10:7818–7829. https://doi.org/10.1021/nn201817b
Kouotou PM, Vieker H, Tian ZY, Ngamou PHT, Kasmi AE, Beyer A, Golzhauser A, Hoinghaus KK (2014) Structure-activity relation of spinel-type Co-Fe oxides for low-temperature CO oxidation. Catal Sci Technol 4:3359–3367. https://doi.org/10.1039/c4cy00463a
Zhang XD, Hou FL, Li HX, Yang Y, Wang YX, Liu N, Yang Y (2018) A strawsheave-like metal organic framework Ce-BTC derivative containing high specific surface area for improving the catalytic activity of CO oxidation reaction. Microporous Mesoporous Mater 259:211–219. https://doi.org/10.1016/j.micromeso.2017.10.019
Rajasree R (2004) Transient kinetics of carbon monoxide oxidation by oxygen over supported palladium/ceria/zirconia three-way catalysts in the absence and presence of water and carbon dioxide. J Catal 22:36–43. https://doi.org/10.1016/j.jcat.2003.12.014
Yang H, Lv K, Zhu JJ, Li Q, Tang DG, Ho WK, Li M, Carabineiro SAC (2017) Effect of mesoporous g-C3N4 substrate on catalytic oxidation of CO over Co3O4. Appl Surf Sci 401:333–340. https://doi.org/10.1016/j.apsusc.2016.12.238
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 41673093, 41473108, 41773128, 41573096, 51508327).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, N., Tao, P., Jing, C. et al. A facile fabrication of nanoflower-like Co3O4 catalysts derived from ZIF-67 and their catalytic performance for CO oxidation. J Mater Sci 53, 15051–15063 (2018). https://doi.org/10.1007/s10853-018-2696-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2696-3