Abstract
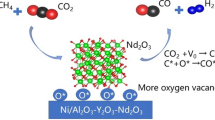
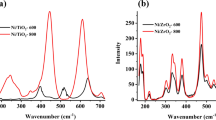
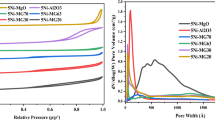
A series of Ni-modified Mo2C catalysts with La2O3-stabilized ZrO2 supports were synthesized via template method coupled with incipient wetness impregnation and temperature-programmed carbonization. The catalyst was tested in dry reforming of methane (DRM) reaction at the temperature range from 973 to 1173 K in a fixed-bed quartz reactor under atmospheric pressure. XRD, Raman, BET, H2-TPD, SEM, EDS and TEM were conducted to characterize the phase constitution, the pore structure, the morphology and the crystal structure of the catalysts. Ni–Mo2C nanoparticles of ~ 3 nm diameter were obtained for these catalysts, and the La2O3–ZrO2 supports exhibited a cubic lattice structure with a sheet shape, which is believed to contain an abundant of oxygen vacancies. And the synergistic interaction between the oxygen vacancy in La–Zr–O solid solution and the Ni–Mo2C particles could decrease the apparent activation energy of CO2. Both of them are beneficial for accelerating DRM reaction. CH4 conversion of the best specimen catalyst reaches 94% at 1173 K, and the catalyst maintains its stability after 100-h reaction. The superior catalytic activity of NMLZ-7.5 is attributed to the improved BET surface area (33.2 m2/g), smaller crystallites, grain boundaries and oxidation–carbonization cycle of Ni–Mo2C.






Similar content being viewed by others
References
Sokolov S, Kondratenko EV, Pohl MM, Rodemerck U (2013) Effect of calcination conditions on time on-stream performance of Ni/La2O3–ZrO2 in low-temperature dry reforming of methane. Int J Hydrogen Energy 38:16121–16132
Claridge JB, York APE, Brungs AJ, Marquez-Alvarez C, Sloan J, Tsang SC, Green MLH (1998) New catalysts for the conversion of methane to synthesis gas: molybdenum and tungsten carbide. J Catal 180:85–100
Kumar N, Shojaee M, Spivey JJ (2015) Catalytic bi-reforming of methane: from greenhouse gases to syngas. Curr Opin Chem Eng 9:8–15
Zhou X, Huang WY, Liu J, Wang HH, Li Z (2017) Quenched breathing effect, enhanced CO2 uptake and improved CO2/CH4 selectivity of MIL-53(Cr)/graphene oxide composites. Chem Eng Sci 167:98–104
Abdollahifar M, Haghighi M, Sharifi M (2015) Dry reforming of methane over nanostructured Co/Y catalyst for hydrogen production: effect of ultrasound irradiation and Co-loading on catalyst properties and performance. Energy Convers Manag 103:1101–1112
Al-Doghachi FAJ, Rashid U, Taufiq-Yap YH (2016) Investigation of Ce(III) promoter effects on the tri-metallic Pt, Pd, Ni/MgO catalyst in dry-reforming of methane. RSC Adv 6:10372–10384
Stroud T, Smith TJ, Le Sache E, Santos JL, Centeno MA, Arellano-Garcia H, Odriozola JA, Reina TR (2018) Chemical CO2 recycling via dry and bi reforming of methane using Ni–Sn/Al2O3 and Ni–Sn/CeO2–Al2O3 catalysts. Appl Catal B Environ 224:125–135
Wurzel T, Malcus S, Mleczko L (2000) Reaction engineering investigations of CO2 reforming in a fluidized-bed reactor. Chem Eng Sci 55:3955–3966
Zhang ZL, Verykios XE, MacDonald SM, Affrossman S (1996) Comparative study of carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 and conventional nickel-based catalysts. J Phys Chem 100:744–754
Souza MMVM, Aranda DAG, Schmal M (2001) Reforming of methane with carbon dioxide over Pt/ZrO2/Al2O3 catalysts. J Catal 204:498–511
Munera JF, Irusta S, Cornaglia LM, Lombardo EA, Cesar DV, Schmal M (2007) Kinetics and reaction pathway of the CO2 reforming of methane on Rh supported on lanthanum-based solid. J Catal 245:25–34
Tsipouriari VA, Verykios XE (2001) Kinetic study of the catalytic reforming of methane with carbon dioxide to synthesis gas over Ni/La2O3 catalyst. Catal Today 64:83–90
Nguyen TH, Lamacz A, Krzton A, Ura A, Chalupka K, Nowosielska M, Rynkowski J, Djega-Mariadassou G (2015) Partial oxidation of methane over Ni-0/La2O3 bifunctional catalyst II: global kinetics of methane total oxidation, dry reforming and partial oxidation. Appl Catal B Environ 165:389–398
Goula MA, Charisiou ND, Siakavelas G, Tzounis L, Tsiaoussis I, Panagiotopoulou P, Goula G, Yentekakis IV (2017) Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int J Hydrogen Energy 42:13724–13740
Lemonidou AA, Vagia EC, Lercher JA (2013) Acetic acid reforming over Rh supported on La2O3/CeO2–ZrO2: catalytic performance and reaction pathway analysis. ACS Catal 3:1919–1928
Evans PA, Stevens R, Binner JGP (1984) Quantitative X-ray-diffraction analysis of polymorphic mixes of pure zirconia. Trans J Br Ceram Soc 83:39–43
Mccullough JD, Trueblood KN (1959) The crystal structure of baddeleyite (Monoclinic Zro2). Acta Crystallogr A 12:507–511
Scott HG (1975) Phase relationships in zirconia–yttria system. J Mater Sci 10:1527–1535. https://doi.org/10.1007/BF01031853
Nilufer IB, Gokce H, Muhaffel F, Ovecoglu ML, Cimenoglu H (2016) The effect of La2O3 on the microstructure and room temperature mechanical properties of t-ZrO2. Ceram Int 42:9443–9447
Si J, Liu GL, Liu JG, Zhao L, Li SS, Guan Y, Liu Y (2016) Ni nanoparticles highly dispersed on ZrO2 and modified with La2O3 for CO methanation. RSC Adv 6:12699–12707
Wang SC, Xie H, Lin YY, Poeppelmeier KR, Li T, Winans RE, Cui YR, Ribeiro FH, Canlas CP, Elam JW, Zhang HB, Marshall CL (2016) High thermal stability of La2O3- and CeO2-stabilized tetragonal ZrO2. Inorg Chem 55:2413–2420
Zheng YN, Li KZ, Wang H, Wang YH, Tian D, Wei YG, Zhu X, Zeng CH, Luo YM (2016) Structure dependence and reaction mechanism of CO oxidation: a model study on macroporous CeO2 and CeO2–ZrO2 catalysts. J Catal 344:365–377
Kilner JA, Drennan J, Dennis P, Steele BCH (1981) A study of anion transport in bismuth based oxide systems by electrical-conductivity and secondary ion mass-spectroscopy (Sims). Solid State Ionics 5:527–530
Sokolov S, Kondratenko EV, Pohl MM, Barkschat A, Rodemerck U (2012) Stable low-temperature dry reforming of methane over mesoporous La2O3–ZrO2 supported Ni catalyst. Appl Catal B Environ 113:19–30
Chen YZ, Liaw BJ, Kao CF, Kuo JC (2001) Yttria-stabilized zirconia supported platinum catalysts (Pt/YSZs) for CH4/CO2 reforming. Appl Catal a Gen 217:23–31
Guell BM, da Silva IMT, Seshan K, Lefferts L (2009) Sustainable route to hydrogen—design of stable catalysts for the steam gasification of biomass related oxygenates. Appl Catal B Environ 88:59–65
Rostrupnielsen JR, Hansen JHB (1993) Co2-reforming of methane over transition-metals. J Catal 144:38–49
Bradford MCJ, Vannice MA (1996) Catalytic reforming of methane with carbon dioxide over nickel catalysts. 1. Catalyst characterization and activity. Appl Catal A Gen 142:73–96
Zhang SH, Shi C, Chen BB, Zhang YL, Zhu YJ, Qiu JS, Au CT (2015) Catalytic role of beta-Mo2C in DRM catalysts that contain Ni and Mo. Catal Today 258:676–683
Wu HD, Yin Y, Liu HZ, Liu TS, Zhao L (2014) Production of hydrogen from ethanol steam reforming over Ni–Mn/La2O3–ZrO2 Catalyst. Asian J Chem 26:383–388
Zhang ZL, Verykios XE, MacDonald SM, Affrossman S (1996) Comparative study of carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 and conventional nickel-based catalysts. J Phys Chem 100:744–754
Tsipas SA (2010) Effect of dopants on the phase stability of zirconia-based plasma sprayed thermal barrier coatings. J Eur Ceram Soc 30:61–72
Li WZ, Zhao ZK, Ren PP, Wang GR (2015) Effect of molybdenum carbide concentration on the Ni/ZrO2 catalysts for steam-CO2 bi-reforming of methane. Rsc Adv 5:100865–100872
Kislov VR, Skudin VV, Adamu A (2017) New bimetallic Mo2C-WC/Al2O3 membrane catalysts in the dry reforming of methane. Kinet Catal 58:73–80
Shi C, Zhang A, Li X, Zhang S, Zhu A, Ma Y, Au C (2012) Ni-modified Mo2C catalysts for methane dry reforming. Appl Catal A Gen 431:164–170
Yao L, Wang Y, Galvez ME, Hu C, Da Costa P (2018) Ni–Mo2C supported on alumina as a substitute for Ni–Mo reduced catalysts supported on alumina material for dry reforming of methane. C R Chim 21:247–252
Zou H, Chen S, Huang J, Zhao Z (2017) Effect of impregnation sequence on the catalytic performance of NiMo carbides for the tri-reforming of methane. Int J Hydrogen Energy 42:20401–20409
Darujati ARS, Thomson WJ (2005) Stability of supported and promoted-molybdenum carbide catalysts in dry-methane reforming. Appl Catal A Gen 296:139–147
Gao H, Yao Z, Shi Y, Jia R, Liang F, Sun Y, Mao W, Wang H (2018) Simple and large-scale synthesis of beta-phase molybdenum carbides as highly stable catalysts for dry reforming of methane. Inorg Chem Front 5:90–99
Gao H, Yao Z, Shi Y, Wang S (2018) Improvement of the catalytic stability of molybdenum carbide via encapsulation within carbon nanotubes in dry methane reforming. Catal Sci Technol 8:697–701
Liang P, Gao H, Yao Z, Jia R, Shi Y, Sun Y, Fan Q, Wang H (2017) Simple synthesis of ultrasmall beta-Mo2C and alpha-MoC1−x nanoparticles and new insights into their catalytic mechanisms for dry reforming of methane. Catal Sci Technol 7:3312–3324
Guo J, Zhang AJ, Zhu AM, Xu Y, Au CT, Shi C (2010) A carbide catalyst effective for the dry reforming of methane at atmospheric pressure. ACS Symposium 1056:181–196
Shi C, Zhang AJ, Li XS, Zhang SH, Zhu AM, Ma YF, Au CT (2012) Ni-modified Mo2C catalysts for methane dry reforming. Appl Catal A Gen 431:164–170
Zhang AJ, Zhu AM, Chen BB, Zhang SH, Au CT, Shi CA (2011) In-situ synthesis of nickel modified molybdenum carbide catalyst for dry reforming of methane. Catal Commun 12:803–807
LaMont DC, Thomson WJ (2005) Dry reforming kinetics over a bulk molybdenum carbide catalyst. Chem Eng Sci 60:3553–3559
Li WZ, Zhao ZK, Ren PP, Wang GR (2015) Effect of molybdenum carbide concentration on the Ni/ZrO2 catalysts for steam-CO2 bi-reforming of methane. RSC Adv 5:100865–100872
He Z, Yang M, Wang XQ, Zhao Z, Duan AJ (2012) Effect of the transition metal oxide supports on hydrogen production from bio-ethanol reforming. Catal Today 194:2–8
Yuan K, Feng C, Gan X, Yu Z, Wang X, Zhu L et al (2016) Fabrication of La2Zr2O7, ceramic fibers via electrospinning method using different La2O3 precursors. Ceram Int 42:16633–16639
Orera A, Larraz G, Sanjuán ML (2013) Spectroscopic study of the competition between dehydration and carbonation effects in La2O3-based materials. J Eur Ceram Soc 33:2103–2110
Junior ES, Antonio SG, Longo E (2017) Synthesis and structural evolution of partially and fully stabilized ZrO2 from a versatile method aided by microwave power. Ceram Int 44:3517–3522
Tao W, Cheng GW, Yao WL, Lu XG, Zhu QH, Li GS, Zhou ZF (2014) Syngas production by CO2 reforming of coke oven gas over Ni/La2O3–ZrO2 catalysts. Int J Hydrogen Energy 39:18650–18658
Ma YF, Guan GQ, Phanthong P, Hao XG, Huang W, Tsutsumi A, Kusakabe K, Abudula A (2014) Catalytic activity and stability of nickel-modified molybdenum carbide catalysts for steam reforming of methanol. J Phys Chem C 118:9485–9496
Tang C, Wang HF, Chen X, Li BQ, Hou TZ, Zhang BS, Zhang Q, Titirici MM, Wei F (2016) Oxygen electrocatalysis: topological defects in metal-free nanocarbon for oxygen electrocatalysis. Adv Mater 28:6845
Amsif M, Magraso A, Marrero-Lopez D, Ruiz-Morales JC, Canales-Vazquez J, Nunez P (2012) Mo-substituted lanthanum tungstate La28−yW4+yO54+delta: a competitive mixed electron–proton conductor for gas separation membrane applications. Chem Mater 24:3868–3877
Antonio RP, Philomena S, Sergio T, Gianfranco P (2017) Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. RSC Adv 55:6493–6513
He H, Dai H, Au CT (2004) Defective structure, oxygen mobility, oxygen storage capacity, and redox properties of RE-based (RE = Ce, Pr) solid solutions. Catal Today 90:245–254
Chen DK, He DD, Lu JC, Zhong LP, Liu F, Liu JP, Yu J, Wan GP, He SF, Luo YM (2017) Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H-2-TPR characterization. Appl Catal B Environ 218:249–259
Sun FM, Yan CF, Wang ZD, Guo CQ, Huang SL (2015) Ni/Ce–Zr–O catalyst for high CO2 conversion during reverse water gas shift reaction (RWGS). Int J Hydrogen Energy 40:15985–15993
Nguyen TH, Lamacz A, Krzton A, Liszka B, Djega-Mariadassou G (2016) Partial oxidation of methane over Ni-0/La2O3 bifunctional catalyst III. Steady state activity of methane total oxidation, dry reforming, steam reforming and partial oxidation. Sequences of elementary steps. Appl Catal B Environ 182:385–391
Yan ZX, Wang HE, Zhang MP, Jiang ZF, Jiang TS, Xie JM (2013) Pt supported on Mo2C particles with synergistic effect and strong interaction force for methanol electro-oxidation. Electrochim Acta 95:218–224
Nikoo MK, Amin NAS (2011) Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process Technol 92:678–691
Zhao ZK, Ren PP, Li WZ, Miao BY (2017) Effect of mineralizers for preparing ZrO2 support on the supported Ni catalyst for steam-CO2 bi-reforming of methane. Int J Hydrogen Energy 42:6598–6609
Li WZ, Zhao ZK, Jiao YH (2016) Dry reforming of methane towards CO-rich hydrogen production over robust supported Ni catalyst on hierarchically structured monoclinic zirconia nanosheets. Int J Hydrogen Energy 41:17907–17921
Duan YP, Shang RS, Zhong XY, Xie W, Wang XY, Huang LH (2016) In-situ synthesis of Ni–Mo2C/Al2O3 catalysts for dry reforming of methane. Int J Hydrogen Energy 41:21955–21964
Nguyen TH, Lamacz A, Krzton A, Ura A, Chalupka K, Nowosielska M, Rynkowski J, Djega-Mariadassou G (2015) Partial oxidation of methane over Ni-0/La2O3 bifunctional catalyst II: global kinetics of methane total oxidation, dry reforming and partial oxidation. Appl Catal B Environ 165:389–398
Lemonidou AA, Vasalos IA (2002) Carbon dioxide reforming of methane over 5 wt% Ni/CaO–Al2O3 catalyst. Appl Catal A Gen 228:227–235
Bradford MCJ, Vannice MA (1999) The role of metal-support interactions in CO2 reforming of CH4. Catal Today 50:87–96
Bradford MCJ, Vannice MA (1997) Metal-support interactions during the CO2 reforming of CH4 over model TiOx/Pt catalysts. Catal Lett 48:31–38
Acknowledgements
The authors are grateful to the Natural Science Foundation of Guangdong Province (Project No. 2015A030312007), National Natural Science Foundation of China (Project No. 51576201), Key Lab of Renewable Energy Foundation of Chinese Academy of Sciences (Project Nos. Y707j81001, Y609JK1001) and Innovation R&D Team of Dongguan City (2014607117).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tao, Q., Wang, Z., Jayasundera, B. et al. Enhanced catalytic activity of Ni–Mo2C/La2O3–ZrO2 bifunctional catalyst for dry reforming of methane. J Mater Sci 53, 14559–14572 (2018). https://doi.org/10.1007/s10853-018-2642-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2642-4