Abstract
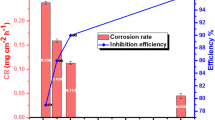
In this work, two imidazolium-based ionic liquids 1-(2-aminoethyl)-1-dodecyl-2-methyl-4,5-dihydro-1H-imidazol-1-ium chloride ([ADMDI]Cl) and 1-(2-aminoethyl)-1-dodecyl-2-(trifluoromethyl)-4,5-dihydro-1H-imidazol-1-ium chloride ([ADTDI]Cl) were synthesized for the first time and their inhibitive effects on the corrosion of mild steel in 0.5 M HCl solution at elevated temperature were investigated by experimental and theoretical methods. The results of weight loss tests show the studied ionic liquids (ILs) can effectively retard the serious corrosion of mild steel and that the greatest efficiencies obtained at 368 K are 86.3% for [ADMDI]Cl and 92.7% for [ADTDI]Cl. Potentiodynamic polarization curves suggest those compounds act as cathodic-type inhibitors and that [ADTDI]Cl has the better inhibiting performance than [ADMDI]Cl, especially at low concentration. It is indicated by the study of scanning electron microscope (SEM) that both the surface morphology and the internal structure of mild steel samples are severely damaged by the corrosive environment at 368 K. However, such bad situation is greatly improved by using IL additives. The higher inhibition efficiency of fluoro-substituted IL proved by experimental results can be explained by theoretical studies. The data of quantum chemical calculations show the introduction of fluoro group can increase the amount of free electrons transferred from IL molecule to the vacant d-orbital of Fe atom and strengthen the dipole–dipole interaction between inhibitor and metal surface. Additionally, molecular dynamics simulations suggest [ADTDI]Cl has the higher adsorption energy than [ADMDI]Cl, which also confirms the better performance of the former as corrosion inhibitor for mild steel in acid environment at elevated temperature.







Similar content being viewed by others
References
Migahed MA, Abdul-Raheim AM, Atta AM, Brostow W (2010) Synthesis and evaluation of a new water soluble corrosion inhibitor from recycled poly (ethylene terphethalate). Mater Chem Phys 121:208–214
Dohare P, Ansari KR, Quraishi MA, Obot IB (2017) Pyranpyrazole derivatives as novel corrosion inhibitors for mild steel useful for industrial pickling process: experimental and quantum chemical study. J Ind End Chem 52:197–210
Hosseini MG, Ehteshamzadeh M, Shahrabi T (2007) Protection of mild steel corrosion with Schiff bases in 0.5 M H2SO4 solution. Electrochim Acta 52:3680–3685
Li YZ, Guo XP, Zhang GA (2017) Inhibition effect of imidazoline inhibitor on the crevice corrosion of N80 carbon steel in the CO2-saturated NaCl solution containing acetic acid. Corros Sci 126:127–141
Padash R, Jamalizadeh E, Jafari AH (2017) Adsorption and corrosion inhibition behavior of aluminium by 2,6-dimethyl pyridine in distilled water. Anti-corros Methods Mater 64:550–554
Yilmaz N, Fitoz A, Ergun Y, Emregul KC (2016) A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino)methyl)phenol as a corrosion inhibitor for mild steel in a highly acidic envmild steelment. Corros Sci 111:110–120
Chaitra TK, Mohana KN, Gurudatt DM (2016) Inhibition activity of new thiazole hydrazones towards mild steel corrosion in acid media by thermodynamic, electrochemical and quantum chemical methods. J Taiwan Inst Chem 67:521–531
Kannan P, Rao TS, Rajendran N (2016) Anti-corrosion behavior of benzimidazoliumtetrafluroborate ionic liquid in acid medium using electrochemical noise technique. J Mol Liq 222:586–595
Ochel A, Lecce DD, Wolff C, Kim G, Carvalho DV, Passerini S (2017) Physicochemical and electrochemical investigations of the ionic liquid N-butyl -N-methyl-pyrrolidinium 4,5-dicyano-2-(trifluoromethyl) imidazole. Electrochim Acta 232:586–595
Ma Y, Han F, Li Z, Xia CG (2016) Corrosion behavior of metallic materials in acidic-functionalized ionic liquids. ACS Sustain Chem Eng 4:633–639
Hanza AP, Naderi R, Kowsari E, Sayebani M (2016) Corrosion behavior of mild steel in H2SO4 solution with 1,4-di[1-methylene-3-methyl imidazolium bromide]-benzene as an ionic liquid. Corros Sci 107:96–106
Guo YY, Xu B, Liu Y, Yang WZ, Yin XS, Chen Y, Le JX, Chen ZH (2017) Corrosion inhibition properties of two imidazolium ionic liquids with hydrophilic tetrafluoroborate and hydrophobic hexafluorophosphate anions in acid medium. J Ind End Chem 56:234–247
Kowsari E, Arman SY, Shahini MH, Zandi H, Ehsani A, Naderi R, PourghasemiHanza A, Mehdipour M (2016) In situ synthesis, electrochemical and quantum chemical analysis of an amino acid-derived ionic liquid inhibitor for corrosion protection of mild steel in 1 M HCl solution. Corros Sci 112:73–85
Qiang YJ, Zhang ST, Guo L, Zheng XW, Xiang B, Chen SJ (2017) Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros Sci 119:68–78
Zhang QB, Hua YX (2009) Corrosion inhibition of mild steel by alkyl imidazolium ionic liquids in hydrochloric acid. Electrochim Acta 54:1881–1887
Sasikumar Y, Adekunle AS, Olasunkanmi LO, Bahadur I, Baskar R, Kabanda MM, Obot IB, Ebenso EE (2015) Experimental, quantum chemical and Monte Carlo simulation studies on the corrosion inhibition of some alkyl imidazolium ionic liquids containing tetrafluoroborate anion on mild steel in acidic medium. J Mol Liq 211:105–118
Yousefi A, Javadian S, Dalir N, Kakemam J, Akbari J (2015) Imidazolium-based ionic liquids as modulators of corrosion inhibition of SDS on mild steel in hydrochloric acid solutions: experimental and theoretical studies. RSC Adv 5:11697–11713
El-Hajjaji F, Messali M, Aljuhani A, Aouad MR, Hammouti B, Belghiti ME, Chauhan DS, Quraishi MA (2018) Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: electrochemical and molecular dynamics simulation studies. J Mol Liq 249:997–1008
Lin XS, Hu XQ, Concepcion JJ, Chen ZF, Liu SB, Meyer TJ, Yang WT (2012) Theoretical study of catalytic mechanism for single-site water oxidation process. PNAS 109:15669–15672
Ochterski JW, Thermochemistry in Gaussian. 2000, Gaussian, Inc
Mendonca G, Costa SN, Freire VN, Cascino P, Correia AN, Lima-Neto P (2017) Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros Sci 115:41–55
Sigircik G, Yildirim D, Tuken T (2017) Synthesis and inhibitory effect of N, N’-bis(1-phenylethanol)ethylenediamine against steel corrosion in HCl Media. Corros Sci 120:184–193
Ramirez-Estrada A, Mena-Cervantes VY, Elizalde I, Manzo-Robledo A, Zamudio-Rivera LS, Nieto-Alvarez DA, Farelas F, Hernandez-altamirano R (2017) Development of a zwitterionic compound derived from β-amino acid as a green inhibitor for CO2 corrosive envmild steelments. ACS Sustain Chem Eng 5:10396–10406
Zhang KG, Yang WZ, Yin XS, Chen Y, Liu Y, Le JX, Xu B (2018) Amino acids modified konjac glucomannan as green corrosion inhibitors for mild steel in HCl solution. Carbohyd Polym 181:191–199
Kumar S, Vashisht H, Olasunkanmi LO, Bahadur I, Verma H, Goyal M, Singh G, Ebenso EE (2017) Polyurethane based triblock copolymers as corrosion inhibitors for mild steel in 0.5 M H2SO4. Ind Eng Chem Res 56:441–456
Olasunkanmi LO, Obot IB, Kabanda MM, Ebenso EE (2015) Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: experimental and theoretical studies. J Phys Chem C 119:16004–16010
Verma C, Olasunkanmi LO, Ebenso EE, Quaishi MA, Obot IB (2016) Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J Phys Chem C 120:11598–11611
Mirzakhanzadeh Z, Kosari A, Moayed MH, Naderi R, Taheri P, Mol JMC (2018) Enhanced corrosion protection of mild steel by the synergetic effect of zinc aluminum polyphosphate and 2-mercaptobenzimidazole inhibitors incorporated in epoxy-polyamide coatings. Corros Sci. 138:372–379
Zhang KG, Yang WZ, Xu B, Liu Y, Yin XS, Chen YZ (2015) Corrosion inhibition of mild steel by bromide-substituted imidazoline in hydrochloric acid. J Taiwan Inst Chem 57:167–174
Zhang KG, Xu B, Yang WZ, Yin XS, Liu Y, Chen YZ (2015) Halogen-substituted imidazoline derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 90:284–295
Xu B, Ji Y, Zhang XQ, Jin XD, Yang WZ, Chen YZ (2016) Experimental and theoretical evaluation of N, N-Bis(2-pyridylmethyl)aniline as a novel corrosion inhibitor for mild steel in hydrochloric acid. J Taiwan Inst Chem 59:526–535
Shen S, Guo XY, Song P, Pan YC, Wang HQ, Wen Y, Yang HF (2013) Phytic acid adsorption on the copper surface: observation of electrochemistry and Raman spectroscopy. Appl Surf Sci 276:167–173
Guzman-Lucero D, Olivares-Xometl O, Martinez-Palou R, Likhanova NV, Dominguez-Aguilar MA, Garibay-Febles V (2011) Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind Eng Chem Res 50:7129–7140
Singh P, Makowska-Janusik M, Slovensky P, Quraishi MA (2016) Nicotinonitriles as green corrosion inhibitors for mild steel in hydrochloric aci: electrochemical, computational and surface morphological studies. J Mol Liq 220:71–81
Zhang KG, Yang WZ, Xu B, Chen Y, Yin XS, Liu Y, Zuo HZ (2018) Inhibitory effect of konjac glucomanan on pitting corrosion of AA5052 aluminium alloy in NaCl solution. J Colloid Interf Sci 517:52–60
Fitoz A, Nazir H, Ozgur M, Emregul E, Emregul KC (2018) An experimental and theoretical approach towards understanding the inhibitive behavior of a nitrile substituted coumarin compound as an effective acidic media inhibitor. Corros Sci 133:451–464
Kannan P, Rao TS, Rajendran N (2018) Improvement in the corrosion resistance of carbon steel in acidic condition using naphthalen-2-ylnaphthalene-2-carboxammide inhibitor. J Colloid Interf Sci 512:618–628
Xu B, Gong WN, Zhang KG, Yang WZ, Liu Y, Yin XS, Shi H, Chen YZ (2015) Theoretical prediction and experimental study of 1-Butyl-2-(4-methylphenyl) benzimidazole as a novel corrosion inhibitor for mild steel in hydrochloric acid. J Taiwan Inst Chem 51:193–200
Singh P, Srivastava V, Quraishi MA (2016) Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: electrochemical, SEM, AFM, and XPS studies. J Mol Liq 216:164–173
Abd-EI-Lateef HM, Abu-Dief AM, Mohamed MAA (2017) Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J Mol Struct 1130:522–542
Ebenso EE, Kabanda MM, Murulana LC, Singh AK, Shukla SK (2012) Electrochemical and quantum chemical investigation of some azine and thiazine dyes as potential corrosion inhibitors for mild steel in hydrochloric acid solution. Ind Eng Chem Res 51:12940–12958
Guo L, Zhu SH, Zhang ST (2015) Experimental and theoretical studies of benzalkonium chloride as an inhibitor for carbon steel corrosion in sulfuric acid. J Ind End Chem 24:174–180
Li W, He Q, Pei C, Hou B (2007) Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim Acta 52:6386–6394
Obot IB, Umoren SA, Gasem ZM, Suleiman R, El Ali B (2015) Theoretical prediction and electrochemical evaluation of vinylimidazole and allylimidazole as corrosion inhibitors for mild steel in 1 M HCl. J Ind Eng Chem 21:1328–1339
Wang D, Xiang B, Liang Y, Song S, Liu C (2014) Corrosion control of copper in 3.5 wt% NaCl solution by domperidone: experimental and theoretical study. Corros Sci 85:77–86
Ongun Yüce A, Dogru Mert B (2014) Kardas G¸ Yazıcı B. Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution. Corros Sci 83:310–316
Okoli CP, Guo QJ, Adewuyi GO (2014) Application of quantum descriptors for predicting adsorption performance of starch and cyclodextrin adsorbents. Carbohyd Polym 101:40–49
Ebenso EE, Arslan T, Kandemirli F, Love I, Ogretir C, Saracoglu M, Umoren SA (2010) Theoretical studies of some sulphonamides as corrosion inhibitors for mild steel in acidic medium. Int J Quantum Chem 110:2614–2634
Mobin M, Rizvi M (2016) Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr Polym 136:384–393
Ebenso EE, Kabanda MM, Arslan T, Saracoglu M, Kandemirli F, Murulana LC, Hammouti B, Quraishi MA, Obot IB, Eddy NO (2012) Quantum chemical investigations on quinoline derivatives as effective corrosion inhibitors for mild steel in acidic medium. J Electrochem Sci 7:5643–5676
Ma Y, Han F, Li Z, Xia CG (2016) Acidic-functionalized ionic liquid as corrosion inhibitor for 304 stainless steel in aqueous sulfuric acid. ACS Sustain Chem Eng 4:5046–5052
Acknowledgement
Financial supports from the National Key R&D Program of China (Grant No. 2017YFC0404100), National Science & Technology Pillar Program during the Twelfth Five-year Plan Period for Seawater Desalination Technology (Grant No. 2015BAB08B00), National Natural Science Foundation of China (Grant No. 21605084) and Project supported by the Natural Science Foundation for Young Scholars of Jiangsu Province, China (Grant No. BK20160983) are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, K., Yang, W., Chen, Y. et al. Enhanced inhibitive performance of fluoro-substituted imidazolium-based ionic liquid for mild steel corrosion in hydrochloric acid at elevated temperature. J Mater Sci 53, 14666–14680 (2018). https://doi.org/10.1007/s10853-018-2616-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2616-6