Abstract
Hybrid-phase-MoS2 (1T@2H-MoS2) nanosheet-coated black TiO2 spheres (1T@2H-MoS2/B-TiO2) were prepared using a hydrothermal method and a chemical reduction method under an argon atmosphere. X-ray diffraction, transmission electron microscope, Raman spectra and X-ray photoelectron spectroscopy indicated the generation of the 1T@2H-MoS2 and black TiO2. The efficiency of the 1T@2H-MoS2/B-TiO2 core–shell structure degradation of rhodamine B (RhB) reached 98.0%. The high photocatalytic activity was due to the presence of 1T-MoS2, which accelerated the electron transfer to participate in the redox reaction. In addition, the presence of B-TiO2 suppressed the undesirable electron–hole recombination. Finally, the synergistic effect between 1T@2H-MoS2 and B-TiO2 promoted the separation of the photoelectron–hole pairs. Moreover, scavenger studies found that the hydroxyl radical (•OH) was the dominant reactive oxygen species in the degradation of RhB under visible light irradiation. The possible growth mechanism and photocatalytic mechanism are discussed in detail. The results of this study will contribute to the improvement in the visible light absorption and photocatalytic degradation of catalysts.
Similar content being viewed by others
Introduction
Photocatalysis is an efficient technology for solar energy conversion and environmental purification and has been widely investigated [1,2,3]. TiO2 is regarded as a promising photocatalyst because of its abundance, non-toxicity, high stability, and environmental friendliness. Nevertheless, its wide bandgap and the rapid recombination rate of the photogenerated electron–holes limit its photocatalytic activity [4, 5]. Continuing efforts have been made to develop approaches to further improve the photocatalytic performance of TiO2 [6,7,8,9]. Recently, Chen et al. reported on the use of black TiO2 nanocrystals with a narrow bandgap of 1.54 eV, which improved the visible light and infrared optical absorption. However, the visible light photocatalytic activity of this sample was not very high [10]. Compared with black TiO2 nanocrystals, the black TiO2 spheres fabricated by Cao et al. exhibited high surface area (~ 100 m2 g−1), a narrow bandgap (2.11 eV), and a visible-light-driven photocatalytic degradation ratio of methyl orange of up to 96% [11]. Therefore, black TiO2 spheres have attracted increased attention because they possess a relatively high photocatalytic degradation rate. In addition, an appropriate semiconductor catalyst coated onto the surface of the TiO2 effectively improves the separation efficiency of the carrier and also provides a larger number of active centers to degrade pollutants [12,13,14]. Liang et al. found that a graphene oxide (GO)/TiO2 hybrid exhibited a quick transfer of electrons from the excited TiO2 particles to GO nanosheets, which improved the efficiency of the degradation although the GO exhibited poor conductivity and electrical properties [15].
MoS2 is a two-dimensional layered material similar to graphene and is a promising candidate for the formation of a heterojunction with black TiO2 spheres due to its high oxidation activity and strong charge-transfer ability [16]. Theoretical and experimental results have shown that the catalytic activity is related to the phase structure of MoS2 [17, 18]. 2H-MoS2 is a semiconductor, and the 1T phase is metallic. 2H-MoS2 with a low transmission capacity and low activity site severely limited its applications in photocatalysis [19]. In contrast, 1T-MoS2 is an ideal cocatalyst candidate because its electron concentration is about 10 times larger than that of 2H-MoS2 and it can accelerate the photogenerated electron transfer and enhance the photocatalytic activity [20,21,22]. For example, Qi et al. reported that the incorporation of 1T-MoS2 into 2H-MoS2 facilitated the efficient electron transfer from the active sites of MoS2 and significantly improved the photocatalytic performance [23]. Zhang et al. prepared metal-phase-assisted 1T@2H-MoSe2 nanostructures to promote the conductivity and improve the in-plane active sites to improve the hydrogen evolution properties [24]. Therefore, the metal phase incorporation into the MoS2 nanosheets (2H phase) is an effective approach for enhancing the photocatalytic activity [25, 26]. Until now, few researchers have reported on the metal 1T-phase incorporation into 2H-MoS2 using black TiO2 spheres for degrading pollutants under visible light irradiation.
In this study, we use black TiO2 spheres as a substrate for the assembly of 1T@2H-MoS2 nanosheets (1T@2H-MoS2/B-TiO2) to form a semiconductor heterojunction core–shell structure for improving the photocatalytic performance. We used black TiO2 spheres as a substrate because the recombination of the photoinduced electrons and holes was inhibited effectively [27]. 1T-MoS2 was used as a cocatalyst to accelerate the electron transfer and promote the oxidation–reduction reaction [28, 29]. Furthermore, owing to the synergistic effect of 1T@2H-MoS2 nanosheets and black TiO2 spheres, the separation ability of the photoinduced electron–hole pairs is enhanced, which results in increased photocatalytic activity [30]. The photocatalytic activity of the synthesized materials was characterized by quantifying the rate of rhodamine B degradation in an aqueous suspension under visible light irradiation. This study provides prospective insights into the design of high-performance photocatalysts for pollutant degradation.
Experimental
Materials
Heptahydrated zinc sulfate (ZnSO4·7H2O), tetrabutyl titanate (TBT), ammonium molybdate ((NH4)6Mo7O24·H2O), and thiourea (CH4N2S) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China) and used without further purification. MoS2 (2H-phase) was purchased from Aladdin Industrial Corporation.
Synthesis of the TiO2 spheres
Synthesis of the TiO2 spheres: The TiO2 spheres were synthesized as described in a previous study [31]. In the synthesis, 1 g of ZnSO4·7H2O was added to 60 ml of ethanol under vigorous stirring for 1 h to form a white suspension; subsequently, 4 ml of TBT was gradually added dropwise to the suspension. The solution was magnetically stirred for about 1 h and then transferred to an autoclave at 200 °C for 24 h and gradually cooled down to room temperature. The final product was obtained from the autoclave by centrifuging thrice with deionized water and ethanol and drying at 70 °C for 6 h.
Synthesis of the 1T@2H-MoS2/B-TiO2 core–shell structures
Synthesis of the 1T@2H-MoS2/B-TiO2 core–shell structures: 1 mmol of (NH4)6Mo7O24·H2O and 15 mmol of CH4N2S were dissolved in 35 ml of distilled water and stirred for few min to form a homogeneous solution. Then, 0.2 g of the obtained TiO2 spheres was added to the mixed solution; after stirring for 30 min, the solution was transferred to a Teflon-lined stainless steel autoclave at 200 °C for 20 h. After cooling to room temperature, the black precipitate was collected, washed with deionized water and ethanol, and dried at 80 °C for 6 h. Subsequently, 0.5 g of the composite sample was ground with 0.5 g of NaBH4 at room temperature for 30 min. Then, the mixtures were transferred into porcelain boats and calcined at 350 °C for 2 h under an Ar atmosphere. After cooling down to room temperature, the 1T@2H-MoS2/B-TiO2 core–shell structures were successfully synthesized. For comparison, the 2H-MoS2/B-TiO2 was also synthesized under the same condition with 2H-MoS2 and TiO2.
Material characterization
The crystal structures and stabilities of the samples were characterized by X-ray diffraction (XRD) (Bruker D8 Advance). Transmission electron microscopy (TEM) (Tecnai G2 F20) was used to study the morphologies and atomic arrangements of the samples. The morphology of the samples was characterized by scanning electron microscopy (SEM) (LEO1430VP). The chemical composition was investigated by energy dispersive spectroscopy (EDS) (LEO1430VP). Raman spectra were obtained on a Raman spectrometer (Bruker, SENTERRA). X-ray photoelectron spectroscopy (XPS) (Thermo ESCALAB 250XI) was used to determine the surface electron states of the samples and the concentration of each phase in the mixed phases. The optical properties were characterized by UV–visible-near-infrared spectroscopy (UV–Vis-NIR) (Hitachi U-3010).
Photocatalytic measurement
The measurements of the photocatalytic activity were carried out at room temperature by the degradation of RhB dyes. A 350 W Xe lamp with a cutoff filter (\( k \ge 420\;{\text{nm}} \)) to remove UV light was served as visible light source. In the photocatalytic test, 40 mL aqueous suspensions of RhB (20 mg L−1) and 10 mg of the sample were placed in a 50-mL beaker. Prior to irradiation, the suspensions were magnetically stirred in the dark for 30 min to establish an adsorption/desorption equilibrium between the RhB and the photocatalyst. Circulating water was used to cool the solution and to prevent solvent evaporation. During the measurement process, about 4 mL of the suspensions were collected at certain time intervals (20 min) for the following analysis after centrifugation. The concentration of RhB was monitored by measuring the absorbance at 554 nm using a UV/Vis spectrometer (Lambda 650 s, Perkin Elmer, USA).
Results and discussion
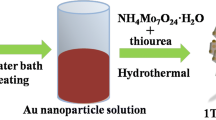
The 1T@2H-MoS2/B-TiO2 core–shell structures are depicted schematically in Fig. 1. First, TiO2 spheres were obtained by a solvothermal method. Then, the obtained TiO2 spheres were added to (NH4)6Mo7O24·H2O and CH4N2S as precursor solutions. Finally, the core–shell structures with the TiO2 spheres as the core and the 1T @2H-MoS2 nanosheets as the shell were prepared at high temperature. After further calcination at 350 °C for 2 h under an Ar atmosphere, the TiO2 spheres changed from white to black, but the morphology remained the same. A SEM image of the 1T@2H-MoS2/B-TiO2 core–shell structures is shown in Fig. 2.
To analyze the morphology and microscopic structure of the as-prepared TiO2 spheres, the MoS2 nanoflowers and 1T@2H-MoS2/B-TiO2 core–shell structures are characterized by SEM. As shown in Fig. 2a, the TiO2 samples are spherical with an average diameter size of 4–5 μm. Figure 2b shows that the 1T@2H-MoS2 nanoflowers are obtained and each petal consists of some MoS2 nanosheets. The 1T@2H-MoS2/B-TiO2 core–shell structures are shown in Fig. 2c. Compared with the TiO2 spheres, it is evident that the MoS2 nanosheets grown on the surface of the B-TiO2 spheres are disorderly and intersect each other and point toward a common center of the sphere to form the core–shell structures. The 1T@2H-MoS2/B-TiO2 core–shell structures are further evaluated by EDS elemental mapping. As shown in Fig. 2d–g, the products are composed of Ti, O, Mo, and S elements; the elements are distributed uniformly, considering the feature of the Ti and O space distribution, which corresponds to the TiO2 spheres (Fig. 2a). Furthermore, the radius of the distribution is larger for the Mo and S than for the Ti and O and the edges of the distribution are relatively rough, which is consistent with our argument that the core consists of TiO2 spheres and the shell is comprised of the MoS2 nanosheets.
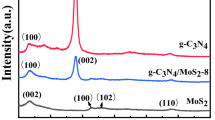
The phase structures of the as-prepared samples are investigated by XRD. For the pure B-TiO2 sample (Fig. 3a), the peaks at 2θ = 25.28°, 37.80°, 48.04°, 53.89°, 62.68°, 68.76°, and 75.02° correspond to the (101), (004), (200), (105), (204), (116), and (215) planes of anatase TiO2 (JCPDS card NO. 21-1272). A major peak at 25.28° can be indexed to the (101) crystal plane of anatase TiO2 corresponding to the interlayer space of 0.35 nm [32]. This will be also confirmed by the TEM, as further discussed below. As shown in Fig. 3b, two diffraction peaks appear at 32.67° and 58.33°, which are assigned to the (100) and (110) lattice planes in the hexagonal phase MoS2 (JCPDS card NO. 37-1492). However, in contrast to the 14.38° and the (002) peak of the 2H-MoS2, the (002)new peak of 1T@2H MoS2 shifts to a lower degree 9.31° and the second-order (004)new diffraction peak with 18.62° simultaneously appears. This change is attributed to the formation of 1T-MoS2 by ammonium ion intercalation, as previously reported [33, 34]. Therefore, this result indicated that the hybrid system of MoS2 was prepared. Figure 3c shows that, after forming the 1T@2H-MoS2/B-TiO2, the diffraction peaks of 1T@2H-MoS2 do not shift and the (101) and (200) diffraction peaks of B-TiO2 are also evident in the XRD pattern. Based on this result, it is confirmed that the 1T@2H-MoS2/B-TiO2 core–shell structures were successfully synthesized.
The Raman spectra of the 1T@2H-MoS2 sample show characteristic peaks at 230 and 332 cm−1, which correlate with the phonon mode of 1T-MoS2. This result is consistent with the results of Shi et al. [29]. Those peaks at around 283, 376, 401, and 450 cm−1 correspond to 2H-MoS2. By contrast, for the 1T@2H-MoS2/B-TiO2 sample, due to the generation of surface stress between 1T-MoS2 and 2H-MoS2 after annealing, the Raman peaks of 1T phase at 230 and 332 cm−1 are significantly reduced. The peak of 2H phase only appeared at 283 cm−1, a phenomenon also observed by Wang et al. [21]. Meanwhile, the 144, 399, 515 and 639 cm−1 diffraction peaks of B-TiO2 [35] are also evident in the Raman spectra. These results demonstrate that the presence of 1T-MoS2, 2H-MoS2 and B-TiO2 in 1T@2H-MoS2/B-TiO2 (Fig. 4).
High-resolution TEM (HRTEM) is performed to visualize the crystal surface structures of the 1T@2H-MoS2/B-TiO2 core–shell structure with lattice fringes. As shown in Fig. 5a, it is clearly observed that the 1T@2H-MoS2 is attached to the surface of the B-TiO2. The yellow rectangle in Fig. 5a is enlarged as shown in Fig. 5b, showing the lattice fringes with an inter-plane spacing of 0.35 nm. In addition, the crystallographic plane of the major exposed surfaces of the B-TiO2 has been determined to be the (101) facet, which is the most dynamically stable crystal facet of the anatase TiO2. The corresponding fast Fourier transform (FFT) pattern (Inset) indicates that the lattice plane of the B-TiO2 is determined to be (101). As shown in Fig. 5c, the magnified white rectangle region in Fig. 5a shows distinct lattice fringes of 0.27 and 0.30 nm, which are attributed to the (100) planes of 1T-MoS2 and the (100) planes of 2H-MoS2, respectively [25]. The 1T-MoS2 and 2H-MoS2 can spot at 30° angular spacing between the hexagonal spot of the 2H-MoS2 assigned to the 1T-phase MoS2 [33]. The blue circled area in Fig. 5c is enlarged in Fig. 5d and evidently displays the trigonal lattice area of the 1T phase; the corresponding structure diagram shows six S atoms surrounding one Mo atom. Figure 5e is the enlarged area of the pink rectangle in Fig. 5c; it displays the common honeycomb lattice area of the trigonal prismatic coordination in the 2H phase and the corresponding structure diagram for the 2H-MoS2 shows that three S atoms surround one Mo atom. The highly magnified images show that the shell of the 1T@2H-MoS2 was formed on the surface of the B-TiO2, which is beneficial to the charge transfer between the B-TiO2 and 1T@2H-MoS2; the synergistic effect between them is beneficial to improve the photocatalytic performance [16].
a Low-magnification TEM images of the 1T@2H-MoS2/B-TiO2 core–shell structures; b HRTEM image of the region enclosed by the yellow rectangle in (a); Inset: FFT of the B-TiO2; c HRTEM image of the region enclosed by the white rectangle in (a); Inset: FFT of the 1T@2H-MoS2; d Image of the region enclosed by the blue circle of (c) and schematic structure of the unit cells of the 1T phase; e Image of the region enclosed by the pink rectangle in (c) and schematic structure of the unit cells of the 2T phase
To analyze the chemical composition and surface chemical bonding of the 1T@2H-MoS2/B-TiO2 core–shell structures, XPS was used. As shown in Fig. 6a, the binding energies of Ti4+ 2p1/2, Ti3+ 2p1/2, Ti4+ 2p3/2, and Ti3+ 2p3/2 are located at 464.3, 463.3, 458.2, and 457.5 eV, respectively. The formation of Ti3+ is due to the redox reaction between TiO2 and NaBH4 [11]. After the reaction with NaBH4, the color of the TiO2 changed from white to black, which is attributed to the generation of oxygen vacancies neighboring the Ti3+. The O 1s states of the samples are shown in Fig. 6b and show two symmetrical peaks located at 531.0 and 529.6 eV, which represent the two different types of O species in the samples. The binding energy of 531.0 eV corresponds to the chemisorbed oxygen, dissociated oxygen, or OH groups on the surface [36]. The shoulder peak observed at 529.6 eV corresponds to the O lattice of TiO2 (Ti–O) [37]. In Fig. 6c, the peaks at around 229.3 and 232.5 eV correspond to the binding energies of the Mo4+ 3d5/2 and Mo4+ 3d3/2 components of 2H-MoS2. The peaks of the blue dotted curves display clear downshifts to a lower energy by ~ 0.7 eV compared with the 2H-MoS2, and they are attributed to the formation of 1T-MoS2, a phenomenon observed in previous studies as well [38]. The other peaks at about 235.8 and 233.5 eV in the 1T@2H-MoS2/B-TiO2 core–shell structures are likely attributed to the existence of a small amount of Mo6+ [39]. For the S 2p peaks (Fig. 6d), the S 2p1/2 and S 2p3/2 peaks shift to 162.7 and 161.6 eV, which are lower than the corresponding binding energies of 2H-MoS2 (163.8 and 162.5 eV). The changes in these peaks can be ascribed to the formation of the 1T-MoS2 [40]. On the other hand, the shift of the Mo 3d and S 2p peaks are attributed to the synergistic effect between the 1T@2H-MoS2 and B-TiO2 [36]. A further analysis of the XPS fitting peak indicated that the ratio of the 1T phase to 1T@2H-MoS2 was 58.6%. The XPS analysis confirms the presence of 1T-MoS2, 2H-MoS2, and B-TiO2 with Ti3+ and oxygen vacancies in the samples.
The UV–Vis absorption spectra of the as-prepared samples are shown in Fig. 7a; the white TiO2 shows a fundamental absorption edge at about 380.0 nm and nearly no absorbance of visible light. Notably, the UV–Vis absorption spectra of the B-TiO2 samples show a broad absorption band in the visible region, which was due to the low-energy photon of the trapped electrons in the localized states of oxygen vacancies associated with Ti3+ [41]. At the same time, the enhancement of the absorption strength is related to the color change of the TiO2 from white to black. Compared with 2H-MoS2, the absorption of 1T@2H-MoS2/B-TiO2 in the visible light region is obviously enhanced. As shown in Fig. 7b, the bandgap values of TiO2, B-TiO2, 2H-MoS2, and 1T@2H-MoS2/B-TiO2 are estimated to be 3.2, 2.7, 1.7, and 1.6 eV, respectively. Among them, the 1T@2H-MoS2/B-TiO2 has narrower bandgap and a better photocatalytic ability, which will be discussed below. The performance of the catalysts is studied by RhB degradation under visible light irradiation as shown in the catalysts is studied by RhB degradation under visible light irradiation as shown in Fig. 7c; a control experiment on the degradation of RhB without photocatalysts was performed and the degradation does not occur for RhB under visible light irradiation. The TiO2 catalyst showed negligible activities, and the degradation was only 1.4% in 120 min because of the large bandgap energy of 3.2 eV [30]. The degradation effects of the B-TiO2 and 1T@2H-MoS2 are obvious, but still poorer than for the 1T@2H-MoS2/B-TiO2 which can degrade 41.6 and 46.8% of RhB. Furthermore, the 1T@2H-MoS2/B-TiO2 composites achieved 98.0% of degradation under visible light irradiation. These results are consistent with those of the UV–Vis results. Photocatalytic degradation of RhB is a pseudo-first-order reaction and its kinetics can be expressed as: ln(C0/C) = k × t, where k is the photocatalytic reaction rate constant; the results are given in Fig. 7d. It can be seen that the k values of TiO2, B-TiO2, 1T@2H-MoS2, and 1T@2H-MoS2/B-TiO2 are 0.007, 0.009, 0.043, and 0.060 min−1, respectively. The k value of 1T@2H-MoS2/B-TiO2 is 6.3 times and 1.4 times higher than the value for B-TiO2 and 1T@2H-MoS2, respectively. Compared with B-TiO2, the photocatalytic degradation rate of 2H-MoS2/B-TiO2 (31.7%) is reduced; the k value of B-TiO2 is 1.1 times higher than the value for 2H-MoS2/B-TiO2. The low rates observed could result from the multilayer structure of the 2H-MoS2 sample, which hinders the charge injection into B-TiO2. Combing with the bandgap of 1T@2H-MoS2/B-TiO2 sample shows that 2H-MoS2 is a narrow bandgap semiconductor, which is beneficial for photon absorption as a photosensitizer. This conclusion is consistent with Muller et al. [42]. To understand the role of active radicals in the 1T@2H-MoS2/B-TiO2 core–shell structures, free radical trapping experiments were carried out and the results are shown in Fig. 7e. Typically, hydroxyl radicals (•OH) and superoxide anions (•O2−) are the possible reactive species in the photocatalytic degradation. Tert-butyl alcohol (TBA), p-benzoquinone (BQ), and disodium ethylenediaminetetraacetic acid (Na2-EDTA) were used as the scavenger for the hydroxyl radicals (•OH), superoxide radicals (•O2−), and holes (h+) [43]. After adding a variety of sacrificial agents, the degradation rate of the RhB was affected to different degrees. Especially, when adding 5 mM TBA, the photocatalytic degradation efficiency of the 1T@2H-MoS2/B-TiO2 was only 46.7%. Therefore, the efficiency of the photocatalytic degradation of RhB is obviously reduced after adding TBA, demonstrating that the photogenerated •OH radical was one of the dominant reactive species due to its highly efficient photocatalytic activity [44]. In addition, the visible light degradation stability of the 1T@2H-MoS2/B-TiO2 is examined by following the degradation of RhB during a five-cycle experiment. After each run, the catalyst was stirred in the dark for 30 min and reused in the next run. As shown in Fig. 7f, the degradation rates remained at 89.3% after 5 cycles. These results indicate that 1T@2H-MoS2/B-TiO2 with a narrow bandgap and high photocatalytic reaction rate results in an excellent and stable photocatalytic activity.
a UV–Vis spectra and b bandgaps of TiO2, B-TiO2, 1T@2H-MoS2/B-TiO2, and 2H-MoS2; c Photocatalytic degradation of RhB for different catalysts under visible light irradiation; d changes in the ln(C/C0) versus visible light irradiation time for different catalysts; e Trapping test of photogenerated holes and radicals with 1T@2H-MoS2/B-TiO2 core–shell structures; f The cycles of degradation of RhB using 1T@2H-MoS2/B-TiO2 as photocatalysts after visible light irradiation for 2 h
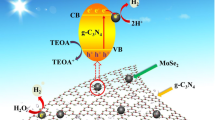
Based on these results, the possible mechanism for the high photocatalytic activity of the 1T@2H-MoS2/B-TiO2 core–shell structures is proposed and is illustrated in Fig. 8. The excellent photocatalytic activity of the 1T@2H-MoS2/B-TiO2 is due to the presence of the 1T phase as a cocatalyst that promotes the electron transfer [45], 2H-MoS2 to serve as a good photosensitizer and B-TiO2 to serve as a substrate. Under simulated visible light irradiation, 2H-MoS2 with a narrow bandgap was excited to generate electron–hole pairs. The electrons are excited from the valence band of 2H-MoS2 to the conduction band, and holes are left in the valence band. Simultaneously, because the 1T phase is a good electron acceptor and has special metal properties, the excited state electrons in the 2H-MoS2 tend to transfer onto 1T-MoS2. B-TiO2 with a high concentration of Ti3+ can induce a vacancy band of electronic states just below the conduction band edge of TiO2 that inhibits electron–hole recombination. During the photocatalytic process, the photogenerated electrons react with oxygen (O2) to form superoxide radicals (•O2−). Meanwhile, the photogenerated holes react with either water (H2O) or hydroxyl ions (OH−) adsorbed onto the catalyst surface, producing hydroxyl radicals (•OH). Consequently, the •OH radicals with strong oxidation ability can decompose the organic compounds. This is consistent with the result of the sacrificial agent experiment, which demonstrated that the photogenerated •OH radical was one of the dominant reactive species for the 1T@2H-MoS2/B-TiO2 core–shell structures’ highly efficient photocatalytic activity.
Therefore, the excellent photocatalytic performance of the 1T@2H-MoS2/B-TiO2 can be ascribed to three factors: (1) the presence of 1T-MoS2, which opens additional electron-transfer channels, thereby achieving better electrons transfer to participate in the redox reaction; (2) the presence of B-TiO2, which provides a lot of Ti3+, more vacancies, and a new energy level lower than the conduction band of TiO2 to suppress the undesirable electron–hole recombination; (3) the synergistic effect of the 1T@2H-MoS2 and B-TiO2, which promote the separation of the photoelectron–hole pairs and enhance the photocatalytic activity under visible light.
Conclusion
In summary, we have successfully synthesized the 1T@2H-MoS2 nanosheet-coated black TiO2 spheres with a simple method. The morphological and structural analyses confirmed the presence of black TiO2 spheres and 1T@2H-MoS2 nanosheets. Significantly, the efficiency of the 1T@2H-MoS2/B-TiO2 core–shell structure degradation of RhB was as high as 98.0%. The exceptional photocatalytic performance of the 1T@2H-MoS2/B-TiO2 core–shell structures was attributed to the 1T-MoS2 functioning as a cocatalyst to improve the electron transfer. Moreover, the B-TiO2 substrate inhibited the electron–hole recombination. Furthermore, the synergistic effect between the 1T@2H-MoS2 and B-TiO2 effectively promoted the separation of the photoelectron–hole pairs. In addition, the photocatalytic reaction rate constant of the 1T@2H-MoS2/B-TiO2 core–shell structures is 6.3 times and 1.4 times higher than the value for B-TiO2 and 1T@2H-MoS2, respectively. The trapping test of the photogenerated holes and radicals of the 1T@2H-MoS2/B-TiO2 core–shell structures confirmed that photogenerated hydroxyl radicals (•OH) played a key role in the degradation of the organic pollutant. Therefore, the 1T@2H-MoS2/B-TiO2 core–shell structures represent promising applications for the degradation of dye pollutants and for the treatment of polluted water.
References
Ge M, Cao C, Huang J, Li S, Chen Z, Zhang KQ, Al-Deyab SS, Lai Y (2016) A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J Mater Chem A 4:6772–6801
Zhang Y, Jiang Z, Huang J, Lim LY, Li W, Deng J, Gong D, Tang Y, Lai Y, Chen Z (2015) Titanate and titania nanostructured materials for environmental and energy applications: a review. RSC Adv 5:79479–79510
Hou J, Zhao H, Huang F, Jing Q, Cao H, Wu Q, Peng S, Cao G (2016) High performance of Mn-doped CdSe quantum dot sensitized solar cells based on the vertical ZnO nanorod arrays. J Power Sources 325:438–445
Han K, Zhang X, Wang H, Liu Y, Cao A (2016) A facile microwaving method to turn titanium oxide into highly active Ti3+ self-doped structure. J Nano Sci Nanotechnol 16:9826–9831
Hamdy MS, Saputera WH, Groenen EJ, Mul G (2014) A novel TiO2 composite for photocatalytic wastewater treatment. J Catal 310:75–83
Ao C, Tian P, Ouyang L, Da G, Xu X, Xu J, Han YF (2016) Dispersing Pd nanoparticles on N-doped TiO2: a highly selective catalyst for H2O2 synthesis. Catal Sci Technol 6:5060–5068
Belver C, Han C, Rodriguez JJ, Dionysiou DD (2017) Innovative W-doped titanium dioxide anchored on clay for photocatalytic removal of atrazine. Catal Today 280:21–28
Reszczyńska J, Grzyb T, Sobczak JW, Lisowski W, Gazda M, Ohtani B, Zaleska A (2015) Visible light activity of rare earth metal doped (Er3+, Yb3+or Er3+/Yb3+) titania photocatalysts. Appl Catal B Environ 163:40–49
Sun Y, Tan J, Lin H, Wang X, Liu J, Li Y, Wang C (2018) A facile strategy for the synthesis of ferroferric oxide/titanium dioxide/molybdenum disulfide heterostructures as a magnetically separable photocatalyst under visible-light. J Colloid Interface Sci 516:138–144
Chen X, Liu L, Yu PY, Mao SS (2011) Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331:746–750
Cao Y, Xing Z, Shen Y, Li Z, Wu X, Yan X, Zou J, Yang S, Zhou W (2017) Mesoporous black Ti3+/N-TiO2 spheres for efficient visible-light-driven photocatalytic performance. Chem Eng J 325:199–207
Zha R, Nadimicherla R, Guo X (2015) Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J Mater Chem A 3:6565–6574
Zhang J, Vasei M, Sang Y, Liu H, Claverie JP (2016) TiO2@Carbon photocatalysts: the effect of carbon thickness on catalysis. ACS Appl Mater Int 8:1903–1912
Yang X, Qin J, Jiang Y, Chen K, Yan X, Zhang D, Li R, Tang H (2015) Fabrication of P25/Ag3PO4/graphene oxide heterostructures for enhanced solar photocatalytic degradation of organic pollutants and bacteria. Appl Catal B Environ 166–167:231–240
Liang Y, Wang H, Sanchez Casalongue H, Chen Z, Dai H (2010) TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res 3:701–705
Liu X, Xing Z, Zhang Y, Li Z, Wu X, Tan S, Yu X, Zhu Q, Zhou W (2017) Fabrication of 3D flower-like black N–TiO2-x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl Catal B Environ 201:119–127
Lukowski MA, Daniel AS, Meng F, Forticaux A, Li L, Jin S (2013) Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J Am Chem Soc 135:10274–10277
Zheng HL, Yang BS, Wang DD, Han RL, Du XB, Yan Y (2014) Tuning magnetism of monolayer MoS2 by doping vacancy and applying strain. Appl Phys Lett 104:132403
Xiang QJ, Yu JG, Jaroniec M (2012) Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J Am Chem Soc 134:6575–6578
Cai L, He J, Liu Q, Yao T, Chen L, Yan W, Hu F, Jiang Y, Zhao Y, Hu T, Sun Z, Wei S (2015) Vacancy-induced ferromagnetism of MoS2 nanosheets. J Am Chem Soc 137:2622–2627
Wang D, Zhang X, Bao S, Zhang Z, Fei H, Wu Z (2017) Phase engineering of a multiphasic 1T/2H MoS2 catalyst for highly efficient hydrogen evolution. J Mater Chem A 5(6):2681–2688
Wang D, Xiao Y, Luo X, Wu Z, Wang YJ, Fang B (2017) Swollen ammoniated MoS2 with 1T/2H hybrid phases for high-rate electrochemical energy storage. ACS Sustain Chem Eng 5(3):2509–2515
Qi Y, Xu Q, Wang Y, Yan B, Ren Y, Chen Z (2016) CO2-induced phase engineering: protocol for enhanced photoelectrocatalytic performance of 2D MoS2 nanosheets. ACS Nano 10:2903–2909
Zhang J, Wang T, Liu P, Liu Y, Ma J, Gao D (2016) Enhanced catalytic activities of metal-phase-assisted 1T@2H-MoSe2 nanosheets for hydrogen evolution. Electrochim Acta 217:181–186
Cai L, Cheng W, Yao T, Huang Y, Tang F, Liu Q, Liu W, Sun Z, Hu F, Jiang Y, Yan W, Wei S (2017) High-content metallic 1T phase in MoS2-based electrocatalyst for efficient hydrogen evolution. J Phys Chem C 121:15071–15077
Gao G, Jiao Y, Ma F, Jiao Y, Waclawik E, Du A (2015) Charge mediated semiconducting-to-metallic phase transition in molybdenum disulfide monolayer and hydrogen evolution reaction in new 1T′ phase. J Phys Chem C 119:13124–13128
Wang D, Su B, Jiang Y, Li L, Ng BK, Wu Z, Liu F (2017) Polytype 1T/2H MoS2 heterostructures for efficient photoelectrocatalytic hydrogen evolution. Chem Eng J 330:102–108
Liu X, Gao S, Xu H, Lou Z, Wang W, Huang B, Dai Y (2013) Green synthetic approach for Ti3+ self-doped TiO2-x nanoparticles with efficient visible light photocatalytic activity. Nanoscale 5:1870–1875
Shi S, Gao D, Xia B, Liu P, Xue D (2015) Enhanced hydrogen evolution catalysis in MoS2 nanosheets by incorporation of a metal phase. J Mater Chem A 3:24414–24421
Jiao Y, Mukhopadhyay A, Ma Y, Yang L, Hafez AM, Zhu H (2018) Ion transport nanotube assembled with vertically aligned metallic MoS2 for high rate lithium-ion batteries. Adv Energy Mater 5:1702779
Wang D, Xu Y, Sun F, Zhang Q, Wang P, Wang X (2016) Enhanced photocatalytic activity of TiO2 under sunlight by MoS2 nanodots modification. Appl Surf Sci 377:221–227
Liu R, Ren F, Yang J, Su W, Sun Z, Zhang L, Wang C (2015) One-step synthesis of hierarchically porous hybrid TiO2 hollow spheres with high photocatalytic activity. Front Mater Sci 10:15–22
Liu C, Chen J, Che H, Huang K, Charpentier PA, Xu WZ, Shi W, Dong H (2017) Construction and enhanced photocatalytic activities of a hydrogenated TiO2 nanobelt coated with CDs/MoS2 nanosheets. RSC Adv 7:8429–8442
Liu Q, Li X, He Q, Khalil A, Liu D, Xiang T, Wu X, Song L (2015) Gram-scale aqueous synthesis of stable few-layered 1T-MoS2: applications for visible-light-driven photocatalytic hydrogen evolution. Small 11:5556–5564
Maitra U, Gupta U, De M, Datta R, Govindaraj A, Rao CNR (2013) Highly effective visible-light-induced H2 generation by single-layer 1T-MoS2 and a nanocomposite of few-layer 2H-MoS2 with heavily nitrogenated graphene. Angew Chem Int Edit 52:13057–13061
Myung ST, Kikuchi M, Yoon CS et al (2013) Black anatase titania enabling ultra high cycling rates for rechargeable lithium batteries. Energy Environ Sci 6(9):2609–2614
Sabarinathan M, Harish S, Archana J, Navaneethan M, Ikeda H, Hayakawa Y (2017) Highly efficient visible-light photocatalytic activity of MoS2–TiO2 mixtures hybrid photocatalyst and functional properties. RSC Adv 7:24754–24763
Lai LL, Wu JM (2015) A facile solution approach to W, N co-doped TiO2 nanobelt thin films with high photocatalytic activity. J Mater Chem A 3:15863–15868
Eda G, Yamaguchi H, Voiry D, Fujita T, Chen M, Chhowalla M (2011) Photoluminescence from chemically exfoliated MoS2. Nano Lett 11:5111–5116
Zhou W, Yin Z, Du Y, Huang X, Zeng Z, Fan Z Liu, Wang H, Zhang J (2013) synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 9:140–147
Liu X, Xing Z, Zhang H et al (2016) Fabrication of 3 D mesoporous black TiO2/MoS2/TiO2 nanosheets for visible-light-driven photocatalysis. Chemsuschem 9:1118–1124
Wei S, Wu R, Jian J et al (2015) Black and yellow anatase titania formed by (H, N)-doping: strong visible-light absorption and enhanced visible-light photocatalysis. Dalton T 44:1534–1538
Mahler B, Hoepfner V, Liao K, Ozin GA (2014) Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: applications for photocatalytic hydrogen evolution. J Am Chem Soc 136(40):14121–14127
Gao W, Wang M, Ran C, Li L (2015) Facile one-pot synthesis of MoS2 quantum dots–graphene–TiO2 composites for highly enhanced photocatalytic properties. Chem Commun 51:1709–1712
Nimbalkar DB, Lo HH, Ramacharyulu PVRK, Ke SC (2016) Improved photocatalytic activity of RGO/MoS2 nanosheets decorated on TiO2 nanoparticles. RSC Adv 6:31661–31667
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 11264037, 51602273, 61464010, and 61604126), Wuhan University of Technology (2018-KF-14), Doctoral fund of Xin Jiang University (BS150219), and Natural Science Foundation of Xinjiang (2017D01C055).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, H., Wu, R., Liu, H. et al. Synthesis of metal-phase-assisted 1T@2H-MoS2 nanosheet-coated black TiO2 spheres with visible light photocatalytic activities. J Mater Sci 53, 10302–10312 (2018). https://doi.org/10.1007/s10853-018-2266-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2266-8