Abstract
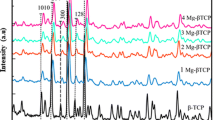
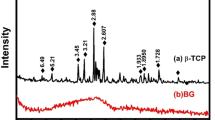
The alpha phase of tricalcium phosphate (α-TCP) can form calcium-deficient hydroxyapatite (CDHA), similar to bone hydroxyapatite. α-TCP can be employed as a biocompatible cement and used as a drug vehicle. Double-setting α-TCP cement composed of acrylamide (α-TCP DS) has better mechanical properties than traditional α-TCP cement. However, no studies on the use of α-TCP DS-based systems for drug release have been reported. In this study, we prepared α-TCP- and α-TCP DS-based samples containing gentamicin sulfate, lidocaine hydrochloride, bupivacaine hydrochloride and levobupivacaine hydrochloride. The properties of the samples were characterized, and the use of the samples as vehicles for these drugs was evaluated. The cements were characterized using X-ray diffraction, Fourier transform infrared spectroscopy, mechanical property tests and apparent porosity evaluation. The drug release in vitro was determined using ultraviolet–visible absorption spectroscopy. For statistical analysis, a confidence interval of 95% with a significance level < 5% was used. Neither the hydrogel nor the drugs interfered with the formation of CDHA, but they increased the mechanical strength of the studied cement. The Peppas–Sahlin model described the mechanisms involved in the drug-release kinetics process, revealing that Fickian diffusion was the main drug-release mechanism with a small influence from the Case II mechanism.






Similar content being viewed by others
References
Carrodeguas RG, De Aza S (2011) α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater 7:3536–3546
Verron E, Gauthier O, Janvier P, Le Guen H, Holopherne D, Cavagna R, Bouler JM (2010) Analgesic properties of calcium phosphate apatite loaded with bupivacaine on postoperative pain. J Biomed Mater Res B Appl Biomater 94:89–96
Ahn M-K, Moon Y-W, Koh Y-H, Kim H-E (2013) Production of highly porous triphasic calcium phosphate scaffolds with excellent in vitro bioactivity using vacuum-assisted foaming of ceramic suspension (VFC) technique. Ceram Int 39:5879–5885
Dos Santos LA, Carrodeguas RG, Rogero SO, Higa OZ, Boschi AO, de Arruda AC (2002) α-Tricalcium phosphate cement: “in vitro” cytotoxicity. Biomaterials 23:2035–2042
Dos Santos LA, de Oliveira LC, Rigo ECS, Carrodeguas RG, Boschi AO, de Arruda ACF (1999) Influence of polymeric additives on the mechanical properties of α-tricalcium phosphate cement. Bone 25:99S–102S
Dos Santos LA, Carrodeguas RG, Boschi AO, Arruda AC (2003) Dual-setting calcium phosphate cement modified with ammonium polyacrylate. Artif Organs 27:412–418
Oriá AP, Neto FAD, Laus JL, dos Santos LA, Piza ET, Brunelli AT, Nishimori CT, de Souza ALG (2006) Evaluation of a double-setting α-tricalcium phosphate cement in eviscerated rabbit eyes. Ophthal Plast Reconstr Surg 22:126–130
Vallet-Regí M, Balas F, Colilla M, Manzano M (2008) Bone-regenerative bioceramic implants with drug and protein controlled delivery capability. Prog Solid State Chem 36:163–191
Aragón J, González R, Fuentes G, Palin L, Croce G, Viterbo D (2012) In vitro release kinetics and physical, chemical and mechanical characterization of a POVIAC®/CaCO3/HAP-200 composite. J Mater Sci Mater Med 23:259–270
Sugo K, Kawashima R, Nakasu M, Nakajima T (2016) Antibiotic elution profile and physical properties of a novel calcium phosphate cement material. J Ceram Soc Jpn 124:954–958
Zalavras CG, Patzakis MJ, Holtom P (2004) Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res 427:86–93
Bond DM, Rudan J, Kobus SM, Adams MA (2004) Depot local anesthetic in polymethylmethacrylate bone cement. A preliminary study. Clin Orthop Relat Res 418:242–245
Giordano V, Rios H, Moreirão M, Giordano M, Amaral NP, Pallottino A, Oliveira S (2007) Ensaio mecânico da resistência ao impacto do cimento ósseo puro e associado a duas drogas anestésicas locais. Rev Bras Ortop 42:225–230
Rang H, Ritter JM, Flower R, Henderson G (2015) Rang and Dale’s pharmacology, 8th edn. Elsevier, Edinburgh
Driessens FCM, Fernández E, Ginebra MP, Boltong MG, Planell JA (1997) Calcium phosphates and ceramic bone cements vs. acrylic cements. Springer, Anales de Química, vol 93, pp 38–43. ISSN : 1130–2283
Espanol M, Perez RA, Montufar EB, Marichal C, Sacco A, Ginebra MP (2009) Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater 5:2752–2762
Fernandes BL, Colpo JC, Manffra EF, Nohama P (2008) Biocompatible glassceramic applied in drug release system. Rev Bras Eng Biomed 24:33–37
Paavola A, Yliruusi J, Kajimoto Y, Kalso E, Wahlström T, Rosenberg P (1995) Controlled release of lidocaine from injectable gels and efficacy in rat sciatic nerve block. Pharm Res 12:1997–2002
Higuchi T (1963) Mechanism of sustained-action medication. theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–1149
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
Peppas NA, Sahlin JJ (1989) A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm 57:169–172
Cirillo G, Spataro T, Curcio M, Spizzirri UG, Nicoletta FP, Picci N, Iemma F (2015) Tunable thermo-responsive hydrogels: synthesis, structural analysis and drug release studies. Mater Sci Eng C 48:499–510
Duncan J, MacDonald JF, Hanna JV, Shirosaki Y, Hayakawa S, Osaka A, Skakle JMS, Gibson IR (2014) The role of the chemical composition of monetite on the synthesis and properties of α-tricalcium phosphate. Mater Sci Eng C 34:123–129
Machado JLM, Giehl IC, Nardi NB, dos Santos LA (2011) Evaluation of scaffolds based on α-tricalcium phosphate cements for tissue engineering applications. IEEE Trans Bio-med Eng 58:1814–1819
Vasconcellos LA, dos Santos LA (2013) Calcium phosphate cement scaffolds with PLGA fibers. Mater Sci Eng C 33:1032–1040
Hurle K, Christel T, Gbureck U, Claus M, Juergen N, Friedlinde G-N (2016) Reaction kinetics of dual setting α-tricalcium phosphate cements. J Mater Sci Mater Med 27:1–13
Fernandes JM, Coelho WT, Thürmer MB, Vieira RS, Santos LA (2012) Properties of calcium phosfate cement with addition of dispersant and hydrogel. Mater Sci Forum 727–28:1170–1174
Beruto DT, Mezzasalma SA, Capurro M, Botter R, Cirillo P (2000) Use of α-tricalcium phosphate (TCP) as powders and as an aqueous dispersion to modify processing, microstructure, and mechanical properties of polymethylmethacrylate (PMMA) bone cements and to produce bone-substitute compounds. J Biomed Mater Res 49:498–505
Marc Bohner M, Lemaître J, Van Landuyt P, Zambelli PY, Merkle HP, Gander B (1997) Gentamicin-loaded hydraulic calcium phosphate bone cement as antibiotic delivery system. J Pharm Sci 86:565–572
Ginebra MP, Traykova T, Planell JA (2006) Calcium phosphate cements as bone drug delivery systems: a review. J Control Release 113:102–110
Wang F-J, Yang Y-Y, Zhang X-Z, Zhu X, Chung T-S, Moochhala S (2002) Cellulose acetate membranes for transdermal delivery of scopolamine base. Mater Sci Eng C 20:93–100
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
Demarchi CA, Debrassi A, Buzzi FC et al (2014) A magnetic nanogel based on O-carboxymethylchitosan for antitumor drug delivery: synthesis, characterization and in vitro drug release. Soft Matter 10:3441–3450
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colpo, J.C., Pigatto, C., Brizuela, N. et al. Antibiotic and anesthetic drug release from double-setting α-TCP cements. J Mater Sci 53, 7112–7124 (2018). https://doi.org/10.1007/s10853-018-2071-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2071-4