Abstract
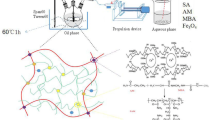
This article presented a versatile and robust adsorbent with both magnetic property and high adsorption capacity on the basis of poly(glycidyl methacrylate)@Fe3O4@diazoresin (PGMA@Fe3O4@DR) magnetic microspheres. The morphology, composition and magnetic properties of the magnetic microspheres were characterized by scanning electron microscope, Fourier transform infrared spectroscopy, thermogravimetric analysis and the hysteresis loop measurements, respectively. The obtained microspheres were applied in the adsorption of dye in the wastewater. The effects of adsorption time, initial dye concentration, pH value and other conditions were explored. Results show that the magnetic microspheres were able to remove more than 98.5% of dyes in water under optimized conditions. The use of diazoresin not only protects the Fe3O4 particles from being corroded at different pH values, but also greatly improves the adsorption capacity.









Similar content being viewed by others
References
Borges GA, Silva LP, Penido JA, de Lemos LR, Mageste AB, Rodrigues GD (2016) A method for dye extraction using an aqueous two-phase system: effect of co-occurrence of contaminants in textile industry wastewater. J Environ Manag 183(1):196–203
Dashamiri S, Ghaedi M, Dashtian K, Rahimi MR, Goudarzi A, Jannesar R (2016) Ultrasonic enhancement of the simultaneous removal of quaternary toxic organic dyes by CuO nanoparticles loaded on activated carbon: central composite design, kinetic and isotherm study. Ultrason Sonochem 31:546
Liang C, Sun S, Li F, Ong Y, Chung T (2014) Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J Membr Sci 469:306–315
Song W, Gao B, Xu X, Xing L, Han S, Duan P, Song W, Jia R (2016) Adsorption–desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater. Bioresour Technol 210:123–130
Jiang D, Amano Y, Machida M (2017) Removal and recovery of phosphate from water by calcium-silicate composites-novel adsorbents made from waste glass and shells. Environ Sci Pollut R 24(9):8210–8218
Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A (2011) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—acid blue 113. J Hazard Mater 186(1):891–901
Bedin KC, Martins AC, Cazetta AL, Pezoti O, Almeida VC (2016) KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal. Chem Eng J 286:476–484
Fernandez ME, Nunell GV, Bonelli PR, Cukierman AL (2014) Activated carbon developed from orange peels: batch and dynamic competitive adsorption of basic dyes. Ind Crop Prod 62:437–445
Gokce Y, Aktas Z (2014) Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl Surf Sci 313:352–359
Nekouei F, Nekouei S, Tyagi I, Gupta VK (2015) Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J Mol Liq 201:124–133
Fu J, Chen Z, Wang M, Liu S, Zhang J, Zhang J, Han R, Xu Q (2015) Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): kinetics, isotherm, thermodynamics and mechanism analysis. Chem Eng J 259:53–61
Meng L, Chan Y, Wang H, Dai Y, Wang X, Zou J (2016) Recycling of iron and silicon from drinking water treatment sludge for synthesis of magnetic iron oxide@SiO2 composites. Environ Sci Pollut R 23(6):5122–5133
Wu H, Shi Y, Guo X, Zhao S, Du J, Jia H, He L, Du L (2016) Determination and removal of sulfonamides and quinolones from environmental water samples using magnetic adsorbents. J Sep Sci 39(22):4398–4407
Zhang Y, Shen S, Wang S, Huang J, Su P, Wang Q, Zhao B (2014) A dual function magnetic nanomaterial modified with lysine for removal of organic dyes from water solution. Chem Eng J 239:250–256
Shariati S, Faraji M, Yamini Y, Rajabi AA (2011) Fe3O4 magnetic nanoparticles modified with sodium dodecyl sulfate for removal of safranin O dye from aqueous solutions. Desalination 270(1–3):160–165
Yan H, Li H, Yang H, Li A, Cheng R (2013) Removal of various cationic dyes from aqueous solutions using a kind of fully biodegradable magnetic composite microsphere. Chem Eng J 223:402–411
Amara D, Grinblat J, Margel S (2010) Synthesis of magnetic iron and iron oxide micrometre-sized composite particles of narrow size distribution by annealing iron salts entrapped within uniform porous poly(divinylbenzene) microspheres. J Mater Chem 20(10):1899–1906
Zhang Y, Su P, Huang J, Wang Q, Zhao B (2015) A magnetic nanomaterial modified with poly-lysine for efficient removal of anionic dyes from water. Chem Eng J 262:313–318
Zhang C, Qiu L, Ke F, Zhu Y, Yuan Y, Xu G, Jiang X (2013) A novel magnetic recyclable photocatalyst based on a core–shell metal–organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J Mater Chem A 1(45):14329–14334
Chen H, Deng C, Zhang X (2010) Synthesis of Fe3O4@SiO2@PMMA core–shell–shell magnetic microspheres for highly efficient enrichment of peptides and proteins for MALDI-ToF MS analysis. Angew Chem Int Ed 49(3):607–611
Reddy DHK, Lee S (2013) Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv Colloid Interface 201:68–93
Zhang X, Zeng T, Wang S, Niu H, Wang X, Cai Y (2015) One-pot synthesis of C18-functionalized core–shell magnetic mesoporous silica composite as efficient sorbent for organic dye. J Colloid Interf Sci 448:189–196
Ma H, Li J, Liu W, Miao M, Cheng B, Zhu S (2015) Novel synthesis of a versatile magnetic adsorbent derived from corncob for dye removal. Bioresour Technol 190:13–20
Yu B, Zhang H, Cong H, Gu C, Gao L, Yang B, Usman M (2017) Diazoresin modified monodisperse porous poly(glycidylmethacrylate-co-divinylbenzene) microspheres as the stationary phase for high performance liquid chromatography. New J Chem 41(11):4637–4643
Yu B, Tian C, Cong H, Xu T (2016) Synthesis of monodisperse poly(styrene-codivinylbenzene) microspheres with binary porous structures and application in high-performance liquid chromatography. J Mater Sci 51:5240–5251. https://doi.org/10.1007/s10853-016-9826-6
Yang J, Hao D, Bi C, Su Z, Wang L, Ma G (2010) Rapid synthesis of uniform magnetic microspheres by combing premix membrane emulsification and in situ formation techniques. Ind Eng Chem Res 49(13):6047–6053
Hwang CW, Kwak N, Hwang TS (2013) Preparation of poly(GMA-co-PEGDA) microbeads modified with iminodiacetic acid and their indium adsorption properties. Chem Eng J 226:79–86
Cao TB, Yang SM, Cao J, Zhang MF, Huang CH, Cao WX (2001) Nanoassembly film of carboxylic polyaniline with photosensitive diazoresin and its photoelectric conversion properties. J Phys Chem B 105(48):11941–11944
Han J, Du Z, Zou W, Li H, Zhang C (2014) Fabrication of interfacial functionalized porous polymer monolith and its adsorption properties of copper ions. J Hazard Mater 276:225–231
Zhang J, Xiao H, Yang Y (2015) Preparation of hemicellulose-containing latex and its application as absorbent toward dyes. J Mater Sci 50(4):1673–1678. https://doi.org/10.1007/s10853-014-8728-8
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21675091, 21574072, 21375069), the Natural Science Foundation for Distinguished Young Scientists of Shandong Province (JQ201403), the Taishan Young Scholar Program of Shandong Province (tsqn20161027), the Key Research and Development Project of Shandong Province (2016GGX102028, 2016GGX102039, 2017GGX20111), the Natural Science Foundation of Shandong Province (ZR2017BEE010), the Project of Shandong Province Higher Educational Science and Technology Program (J15LC20), the Scientific Research Foundation for the Returned Overseas Chinese Scholars of State Education Ministry (20111568), the People’s Livelihood Science and Technology Project of Qingdao (166257nsh, 173378nsh), the Innovation Leader Project of Qingdao (168325zhc), the China Postdoctoral Science Foundation (2017M612203), the Postdoctoral Scientific Research Foundation of Qingdao and the First Class Discipline Project of Shandong Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, B., Yang, B., Li, G. et al. Preparation of monodisperse cross-linked poly(glycidyl methacrylate)@Fe3O4@diazoresin magnetic microspheres with dye removal property. J Mater Sci 53, 6471–6481 (2018). https://doi.org/10.1007/s10853-018-2018-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2018-9