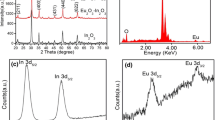
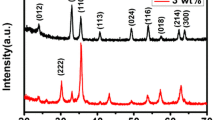
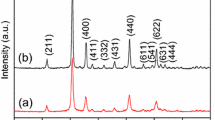
Abstract
Pure and Ho-doped In2O3 nanotubes (NTs) and porous nanotubes (PNTs) were successfully synthesized by conventional electrospinning process and the following calcination at different temperatures. X-ray diffractometry (XRD), thermogravimetric analysis (TGA), Raman spectrometer, energy-dispersive spectroscopy, scanning and transmission electron microscopy were carefully used to investigate the morphologies, structures and chemical compositions of these samples. Their sensing properties toward ethanol gas were studied. Compared with pure In2O3 NTs (response value is 17), pure In2O3 PNTs (response value is 20) demonstrated enhanced sensing characteristics. What’s more, the response of Ho-doped In2O3 PNTs sensors to 100 ppm ethanol was up to 60 at 240 °C, which increased three times more than that of the pure In2O3 PNTs. Additionally, the minimum concentration for ethanol was 200 ppb (response value is 2). The increased gas-sensing ability was attributed not only to the hollow and porous structure, but to the Ho dopant. Furthermore, Ho-doped In2O3 PNTs enable sensor to discriminate between ethanol and the other gas distinctly, particularly acetone that is usually indistinguishable from ethanol. Also, by analyzing XRD, TGA and Raman spectrometer, a possible formation mechanism of porous nanotubes and sensing mechanism were put forward.













Similar content being viewed by others
References
Guo L, Shen X, Zhu G, Chen K (2011) Sens Actuators B Chem 155:752. https://doi.org/10.1016/j.snb.2011.01.042
Wagner T, Haffer S, Weinberger C, Klaus D, Tiemann M (2013) Chem Soc Rev 42:4036. https://doi.org/10.1039/C2CS35379B
Katoch A, Abideen ZU, Kim J-H, Kim SS (2016) Sens Actuators B Chem 232:698. https://doi.org/10.1016/j.snb.2016.04.013
Katoch A, Choi S-W, Kim SS (2014) Nanotechnology 25:455504. https://doi.org/10.1088/0957-4484/25/45/455504
Espinosa EH, Ionescu R, Bittencourt C et al (2007) Thin Solid Films 515:8322. https://doi.org/10.1016/j.tsf.2007.03.017
Kaniyoor A, Imran Jafri R, Arockiadoss T, Ramaprabhu S (2009) Nanoscale 1:382. https://doi.org/10.1039/b9nr00015a
Qian LH, Wang K, Li Y, Fang HT, Lu QH, Ma XL (2006) Mater Chem Phys 100:82. https://doi.org/10.1016/j.matchemphys.2005.12.009
Feng C, Li W, Li C et al (2012) Sens Actuators B Chem 166–167:83. https://doi.org/10.1016/j.snb.2011.12.083
Okamoto A, Shibasaki I (2003) J Cryst Growth 251:560. https://doi.org/10.1016/S0022-0248(02)02448-X
Bloor LG, Manzi J, Binions R et al (2012) Chem Mater 24:2864. https://doi.org/10.1021/cm300596c
Chikhale LP, Patil JY, Rajgure AV, Shaikh FI, Mulla IS, Suryavanshi SS (2014) Ceram Int 40:2179. https://doi.org/10.1016/j.ceramint.2013.07.136
Habibzadeh S, Khodadadi AA, Mortazavi Y (2010) Sens Actuators B Chem 144:131. https://doi.org/10.1016/j.snb.2009.10.047
Qin W, Xu L, Song J, Xing R, Song H (2013) Sens Actuators B Chem 185:231. https://doi.org/10.1016/j.snb.2013.05.001
Che G, Lakshmi BB, Martin CR, Fisher ER, Ruoff RS (1998) Chem Mater 10:260
Tao X, Sun L, Li Z, Zhao Y (2009) Nanoscale Res Lett 5:383. https://doi.org/10.1007/s11671-009-9493-5
Lakshmi BB, Patrissi CJ, Martin CR (1997) Chem Mater 9:2544. https://doi.org/10.1021/cm970268y
Xu L, Song H, Dong B, Wang Y, Chen J, Bai X (2010) Inorg Chem 49:10590. https://doi.org/10.1021/ic101602a
Xu L, Dong B, Wang Y, Bai X, Liu Q, Song H (2010) Sens Actuators B Chem 147:531. https://doi.org/10.1016/j.snb.2010.04.003
Huang Z-M, Zhang YZ, Kotaki M, Ramakrishna S (2003) Compos Sci Technol 63:2223. https://doi.org/10.1016/S0266-3538(03)00178-7
Azhari SJ, Diab MA (1998) Polym Degrad Stab 60:253. https://doi.org/10.1016/S0141-3910(97)00073-6
Chen W-S, Huang D-A, Chen H-C et al (2009) Cryst Growth Des 9:4070. https://doi.org/10.1021/cg900297q
Enhanced acetone sensing properties of Eu-In2O3 nanotubes with bumps (2016) Institution of Engineering and Technology, http://digital-library.theiet.org/content/journals/10.1049/mnl.2016.0235
Kumar M, Singh VN, Singh F, Lakshmi KV, Mehta BR, Singh JP (2008) Appl Phys Lett 92:171907. https://doi.org/10.1063/1.2910501
Olivia MB, Ariano DR, Cleocir JD, Alexandre JCL, Edson RL, Adenilson JC (2010) J Phys D Appl Phys 43:045401
Gan J, Lu X, Wu J et al (2013) Sci Rep 3:1021. https://doi.org/10.1038/srep01021
Dayal P, Kyu T (2006) J Appl Phys 100:043512
Cho JS, Kang YC (2015) Small 11:4673
Tu KN, Gösele U (2005) Appl Phys Lett 86:093111
Yanping L, Zhihong L, Yuhu L, Zhiyong L, Qihou L (2014) Chin J Nonferrous Metals 24:221
Shaalan NM, Rashad M, Abdel-Rahim MA (2016) Mater Sci Semicond Process 56:260. https://doi.org/10.1016/j.mssp.2016.09.007
Lim SK, Hwang S-H, Chang D, Kim S (2010) Sens Actuators B Chem 149:28. https://doi.org/10.1016/j.snb.2010.06.039
Gao L, Cheng Z, Xiang Q, Zhang Y, Xu J (2015) Sens Actuators B Chem 208:436. https://doi.org/10.1016/j.snb.2014.11.053
Zhang L-h, Wang S-l, Liu F-h (2015) J Electron Mater 44:3408. https://doi.org/10.1007/s11664-015-3905-3
Guo X, Zhang J, Ni M, Liu L, Lian H, Wang H (2016) J Mater Sci Mater Electron 27:11262. https://doi.org/10.1007/s10854-016-5247-1
Yu F, Wu Y, Ma J, Zhang C (2013) J Environ Sci 25:195. https://doi.org/10.1016/S1001-0742(12)60023-0
Zhao C, Zhang G, Han W et al (2013) Cryst Eng Commun 15:6491. https://doi.org/10.1039/C3CE40962G
Bagheri M, Khodadadi AA, Mahjoub AR, Mortazavi Y (2015) Sens Actuators B Chem 220:590. https://doi.org/10.1016/j.snb.2015.06.007
Anand K, Kaur J, Singh RC, Thangaraj R (2017) Chem Phys Lett 670:37. https://doi.org/10.1016/j.cplett.2016.12.057
Montazeri A, Jamali-Sheini F (2017) Sens Actuators B Chem 242:778. https://doi.org/10.1016/j.snb.2016.09.181
Navale ST, Bandgar DK, Nalage SR et al (2013) Ceram Int 39:6453. https://doi.org/10.1016/j.ceramint.2013.01.074
Sun P, Cai Y, Du S et al (2013) Sens Actuators B Chem 182:336. https://doi.org/10.1016/j.snb.2013.03.019
Franke ME, Koplin TJ, Simon U (2006) Small 2:36. https://doi.org/10.1002/smll.200500261
Singh S, Singh A, Yadav BC, Dwivedi PK (2013) Sens Actuators B Chem 177:730. https://doi.org/10.1016/j.snb.2012.11.096
Hamedani NF, Mahjoub AR, Khodadadi AA, Mortazavi Y (2012) Sens Actuators B Chem 169:67. https://doi.org/10.1016/j.snb.2012.02.074
Miller DR, Akbar SA, Morris PA (2014) Sens Actuators B Chem 204:250. https://doi.org/10.1016/j.snb.2014.07.074
Anand K, Kaur J, Singh RC, Thangaraj R (2016) Ceram Int 42:10957. https://doi.org/10.1016/j.ceramint.2016.03.233
An W, Wu X, Zeng XC (2008) J Phys Chem C 112:5747. https://doi.org/10.1021/jp711105d
Cheng JP, Wang BB, Zhao MG, Liu F, Zhang XB (2014) Sens Actuators B Chem 190:78. https://doi.org/10.1016/j.snb.2013.08.098
Belmonte JC, Manzano J, Arbiol J et al (2006) Sens Actuators B Chem 114:881. https://doi.org/10.1016/j.snb.2005.08.007
Prabhu E, Gnanasekar KI, Ravindran TR, Jayaraman V, Gnanasekaran T (2014) J Electrochem Soc 161:B176. https://doi.org/10.1149/2.0451409jes
Acknowledgements
The work has been supported by the Jilin Provincial Science and Technology Department (No. 20170101199JC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duan, H., Wang, Y., Li, S. et al. Controllable synthesis of Ho-doped In2O3 porous nanotubes by electrospinning and their application as an ethanol gas sensor. J Mater Sci 53, 3267–3279 (2018). https://doi.org/10.1007/s10853-017-1796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1796-9