Abstract
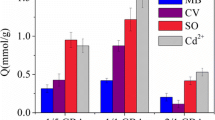
It is a challenge to develop broad-spectrum, high-efficiency, easy-recyclable adsorbents for the removal of water contaminants. Herein, l-cysteine-reduced graphene oxide/poly(vinyl alcohol) (CRG/PVA) ultralight aerogels with good mechanical strength and reusability are prepared via a direct sol–aerogel transition strategy by freeze drying. At optimized composition, the aerogel shows high adsorption efficiency toward both cationic and anionic dyes, overcoming the defect of many traditional adsorbents that usually can only remove one type of organic dyes. The adsorption is proved to involve in π–π interaction between CRG and dyes, endowing the aerogel with universality in adsorbing a wide range of conjugated dyes. Moreover, a remarkable synergetic effect is observed for removal of two oppositely charged dyes from aqueous system, yielding exceptionally high total adsorption capacities surpassing all known adsorbents examined for removing binary dyes. Thus, the CRG/PVA aerogel demonstrates great potential for usage as reusable, high-efficiency, and broad-spectrum adsorbent in water treatment.











Similar content being viewed by others
References
Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3. doi:10.1016/j.watres.2014.08.053
Liu X, Yan L, Yin W et al (2014) A magnetic graphene hybrid functionalized with beta-cyclodextrins for fast and efficient removal of organic dyes. J Mater Chem A 2:12296. doi:10.1039/c4ta00753k
Wan Q, Liu MY, Xie YL et al (2017) Facile and highly efficient fabrication of graphene oxide-based polymer nanocomposites through mussel-inspired chemistry and their environmental pollutant removal application. J Mater Sci 52:504. doi:10.1007/s10853-016-0349-y
Salleh MAM, Mahmoud DK, Karim WAWA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280:1. doi:10.1016/j.desal.2011.07.019
Qu X, Alvarez PJJ, Li Q (2013) Applications of nanotechnology in water and wastewater treatment. Water Res 47:3931. doi:10.1016/j.watres.2012.09.058
Chen A, Li Y, Yu Y et al (2016) Synthesis of mesoporous carbon nanospheres for highly efficient adsorption of bulky dye molecules. J Mater Sci 51:7016. doi:10.1007/s10853-016-9991-7
Liu X, Zhou Y, Nie W, Song L, Chen P (2015) Fabrication of hydrogel of hydroxypropyl cellulose (HPC) composited with graphene oxide and its application for methylene blue removal. J Mater Sci 50:6113. doi:10.1007/s10853-015-9166-y
Liu S, Chen D, Zheng J et al (2015) The sensitive and selective adsorption of aromatic compounds with highly crosslinked polymer nanoparticles. Nanoscale 7:16943. doi:10.1039/c5nr04624f
Yan JJ, Huang YP, Miao YE, Tjiu WW, Liu TX (2015) Polydopamine-coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability. J Hazard Mater 283:730. doi:10.1016/j.jhazmat.2014.10.040
Gad HMH, Daifullah AE-HAM (2007) Impact of surface chemistry on the removal of indigo carmine dye using apricot stone active carbon. Adsorpt Sci Technol 25:327. doi:10.1260/026361707783432588
Lv W, Du M, Ye W, Zheng Q (2015) The formation mechanism of layered double hydroxide nanoscrolls by facile trinal-phase hydrothermal treatment and their adsorption properties. J Mater Chem A 3:23395. doi:10.1039/c5ta05218a
Gil A, Assis FCC, Albeniz S, Korili SA (2011) Removal of dyes from wastewaters by adsorption on pillared clays. Chem Eng J 168:1032. doi:10.1016/j.cej.2011.01.078
Gong JL, Wang B, Zeng GM et al (2009) Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J Hazard Mater 164:1517. doi:10.1016/j.jhazmat.2008.09.072
Yusuf M, Elfghi FM, Zaidi SA, Abdullah EC, Khan MA (2015) Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv 5:50392. doi:10.1039/C5RA07223A
Zhao GX, Jiang L, He YD et al (2011) Sulfonated graphene for persistent aromatic pollutant management. Adv Mater 23:3959. doi:10.1002/adma.201101007
Wu Q, Feng C, Wang C, Wang Z (2013) A facile one-pot solvothermal method to produce superparamagnetic graphene-Fe3O4 nanocomposite and its application in the removal of dye from aqueous solution. Colloids Surf B 101:210. doi:10.1016/j.colsurfb.2012.05.036
Yu JG, Yu LY, Yang H et al (2015) Graphene nanosheets as novel adsorbents in adsorption, preconcentration and removal of gases, organic compounds and metal ions. Sci Total Environ 502:70. doi:10.1016/j.scitotenv.2014.08.077
Parmar KR, Patel I, Basha S, Murthy ZVP (2014) Synthesis of acetone reduced graphene oxide/Fe3O4 composite through simple and efficient chemical reduction of exfoliated graphene oxide for removal of dye from aqueous solution. J Mater Sci 49:6772. doi:10.1007/s10853-014-8378-x
Xue Z, Zhao S, Zhao Z, Li P, Gao J (2016) Thermodynamics of dye adsorption on electrochemically exfoliated graphene. J Mater Sci 51:4928. doi:10.1007/s10853-016-9798-6
Chowdhury S, Balasubramanian R (2014) Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv Colloid Interface Sci 204:35. doi:10.1016/j.cis.2013.12.005
Sharma VK, McDonald TJ, Kim H, Garg VK (2015) Magnetic graphene-carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification. Adv Colloid Interface Sci 225:229. doi:10.1016/j.cis.2015.10.006
Wang H, Yuan X, Zeng G et al (2015) Three dimensional graphene based materials: synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation. Adv Colloid Interface Sci 221:41. doi:10.1016/j.cis.2015.04.005
Zuo L, Zhang Y, Zhang L, Miao Y-E, Fan W, Liu T (2015) Polymer/carbon-based hybrid aerogels: preparation, properties and applications. Materials 8:6806. doi:10.3390/ma8105343
Shen Y, Fang Q, Chen B (2015) Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection. Environ Sci Technol 49:67. doi:10.1021/es504421y
Liu F, Chung S, Oh G, Seo TS (2012) Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl Mater Int 4:922. doi:10.1021/am201590z
Gao HC, Sun YM, Zhou JJ, Xu R, Duan HW (2013) Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl Mater Int 5:425. doi:10.1021/am302500v
Sui Z-Y, Cui Y, Zhu J-H, Han B-H (2013) Preparation of three-dimensional graphene oxide-polyethylenimine porous materials as dye and gas adsorbents. ACS Appl Mater Int 5:9172. doi:10.1021/am402661t
Xu Y, Wu Q, Sun Y, Bai H, Shi G (2010) Three-Dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano 4:7358. doi:10.1021/nn1027104
Bai S, Shen X, Zhong X et al (2012) One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal. Carbon 50:2337. doi:10.1016/j.carbon.2012.01.057
Kim H, Kang SO, Park S, Park HS (2015) Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J Ind Eng Chem 21:1191. doi:10.1016/j.jiec.2014.05.033
Cheng J-S, Du J, Zhu W (2012) Facile synthesis of three-dimensional chitosan–graphene mesostructures for reactive black 5 removal. Carbohydr Polym 88:61. doi:10.1016/j.carbpol.2011.11.065
Xiao J, Lv W, Xie Z, Tan Y, Song Y, Zheng Q (2016) Environmentally friendly reduced graphene oxide as a broad-spectrum adsorbent for anionic and cationic dyes via π–π interactions. J Mater Chem A 4:12126. doi:10.1039/C6TA04119A
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339. doi:10.1021/ja01539a017
Zheng QF, Javadi A, Sabo R, Cai ZY, Gong SQ (2013) Polyvinyl alcohol (PVA)-cellulose nanofibril (CNF)-multiwalled carbon nanotube (MWCNT) hybrid organic aerogels with superior mechanical properties. RSC Adv 3:20816. doi:10.1039/c3ra42321b
Zhang L, Wang Z, Xu C et al (2011) High strength graphene oxide/polyvinyl alcohol composite hydrogels. J Mater Chem 21:10399. doi:10.1039/c0jm04043f
Ye M, Mohanty P, Ghosh G (2014) Morphology and properties of poly vinyl alcohol (PVA) scaffolds: impact of process variables. Mater Sci Eng, C 42:289. doi:10.1016/j.msec.2014.05.029
Tan Y, Song Y, Zheng Q (2012) Hydrogen bonding-driven rheological modulation of chemically reduced graphene oxide/poly(vinyl alcohol) suspensions and its application in electrospinning. Nanoscale 4:6997. doi:10.1039/c2nr32160b
Peng L, Xu Z, Liu Z et al (2015) An iron-based green approach to 1-h production of single-layer graphene oxide. Nat Commun. doi:10.1038/ncomms6716
Xu Y, Sheng K, Li C, Shi G (2010) Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4:4324. doi:10.1021/nn101187z
Kim S, Azuma Y, Kuwahara Y, Ogata T, Kurihara S (2015) Preparation of graphene oxide/polyvinyl alcohol microcomposites and their thermal conducting properties. Mater Lett 139:224. doi:10.1016/j.matlet.2014.10.093
Xue R, Xin X, Wang L et al (2015) A systematic study of the effect of molecular weights of polyvinyl alcohol on polyvinyl alcohol-graphene oxide composite hydrogels. PCCP 17:5431. doi:10.1039/c4cp05766j
Victor-Roman S, Simon-Herrero C, Romero A, Gracia I, Luis Valverde J, Sanchez-Silva L (2015) CNF-reinforced polymer aerogels: influence of the synthesis variables and economic evaluation. Chem Eng J 262:691. doi:10.1016/j.cej.2014.10.026
Alhwaige AA, Herbert MM, Alhassan SM, Ishida H, Qutubuddin S, Schiraldi DA (2016) Laponite/multigraphene hybrid-reinforced poly(vinyl alcohol) aerogels. Polymer 91:180. doi:10.1016/j.polymer.2016.03.077
Zhai T, Zheng Q, Cai Z, Turng L-S, Xia H, Gong S (2015) Poly(vinyl alcohol)/cellulose nanofibril hybrid aerogels with an aligned microtubular porous structure and their composites with polydimethylsiloxane. ACS Appl Mater Interfaces 7:7436. doi:10.1021/acsami.5b01679
Wang Y-T, Liao S-F, Shang K et al (2015) Efficient approach to improving the flame retardancy of poly(vinyl alcohol)/clay aerogels: incorporating piperazine-modified ammonium polyphosphate. ACS Appl Mater Interfaces 7:1780
Shen P, Zhao H-B, Huang W, Chen H-B (2016) Poly(vinyl alcohol)/clay aerogel composites with enhanced flame retardancy. Rsc Adv 6:109809. doi:10.1039/C6RA21689G
Zheng Q, Cai Z, Gong S (2014) Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J Mater Chem A 2:3110. doi:10.1039/c3ta14642a
Fernandez-Merino MJ, Guardia L, Paredes JI et al (2010) Vitamin C Is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J Phys Chem C 114:6426. doi:10.1021/jp100603h
Liang J, Huang Y, Zhang L et al (2009) Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv Funct Mater 19:2297. doi:10.1002/adfm.200801776
Wang Y, Shi ZX, Yin J (2011) Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl Mater Int 3:1127. doi:10.1021/am1012613
Bian Q, Tian H, Wang Y et al (2015) Effect of graphene oxide on the structure and properties of poly(vinyl alcohol) composite films. Polym Sci Ser A 57:836. doi:10.1134/s0965545x15060048
Ali ZI, Ali FA, Hosam AM (2009) Effect of electron beam irradiation on the structural properties of PVA/V2O5 xerogel. Spectroc Acta Pt A-Molec Biomolec Spectr 72:868. doi:10.1016/j.saa.2008.12.013
Mansur HS, Sadahira CM, Souza AN, Mansur AAP (2008) FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater Sci Eng C 28:539. doi:10.1016/j.msec.2007.10.088
Tuinstra F, Koenig JL (1970) Raman spectrum of graphite. J Chem Phys 53:1126. doi:10.1063/1.1674108
Stankovich S, Dikin DA, Piner RD et al (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558. doi:10.1016/j.carbon.2007.02.034
Kundu A, Layek RK, Kuila A, Nandi AK (2012) Highly fluorescent graphene oxide-poly(vinyl alcohol) hybrid: an effective material for specific Au3 + Ion sensors. ACS Appl Mater Int 4:5576. doi:10.1021/am301467z
Chen DZ, Li LD, Guo L (2011) An environment-friendly preparation of reduced graphene oxide nanosheets via amino acid. Nanotechnology. doi:10.1088/0957-4484/22/32/325601
Zhou Q, Gong W, Xie C et al (2011) Removal of Neutral Red from aqueous solution by adsorption on spent cottonseed hull substrate. J Hazard Mater 185:502. doi:10.1016/j.jhazmat.2010.09.029
Ramesha GK, Vijaya Kumara A, Muralidhara HB, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 61:270. doi:10.1016/j.jcis.2011.05.050
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451. doi:10.1016/s0032-9592(98)00112-5
Langmuir I (1918) THE adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361. doi:10.1021/ja02242a004
Freundlich HMF (1906) Concerning adsorption in solutions. Z Phys Chem 57A:385
Zhang J, Shi QQ, Zhang CL, Xu JT, Zhai B, Zhang B (2008) Adsorption of Neutral Red onto Mn-impregnated activated carbons prepared from Typha orientalis. Bioresour Technol 99:8974. doi:10.1016/j.biortech.2008.05.018
Wang S, Wei J, Lv S, Guo Z, Jiang F (2013) Removal of organic dyes in environmental water onto magnetic-sulfonic graphene nanocomposite. CLEAN-Soil Air Water 41:992. doi:10.1002/clen.201200460
Copello GJ, Mebert AM, Raineri M, Pesenti MP, Diaz LE (2011) Removal of dyes from water using chitosan hydrogel/SiO2 and chitin hydrogel/SiO2 hybrid materials obtained by the sol-gel method. J Hazard Mater 186:932. doi:10.1016/j.jhazmat.2010.11.097
Gonzalez JA, Villanueva ME, Piehl LL, Copello GJ (2015) Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: adsorption and desorption study. Chem Eng J 280:41. doi:10.1016/j.cej.2015.05.112
de Oliveira Brito SM, Andrade HMC, Soares LF, de Azevedo RP (2010) Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J Hazard Mater 174:84. doi:10.1016/j.jhazmat.2009.09.020
Zhang J, Zhang P, Zhang S, Zhou Q (2014) Comparative study on the adsorption of tartrazine and indigo carmine onto Maize Cob Carbon. Sep Sci Technol 49:877. doi:10.1080/01496395.2013.863340
Yu L, Wang H, Zhang Y, Zhang B, Liu J (2016) Recent advances in halloysite nanotube derived composites for water treatment. Environ Sci Nano 3:28. doi:10.1039/C5EN00149H
Yasar M, Deligoz H, Guclu G (2011) Removal of indigo carmine and Pb(II) Ion from aqueous solution by polyaniline. Polym-Plast Technol Eng 50:882. doi:10.1080/03602559.2011.551978
Sari MM (2010) Removal of acidic indigo carmine textile dye from aqueous solutions using radiation induced cationic hydrogels. Water Sci Technol 61:2097. doi:10.2166/wst.2010.158
Li M, Wang H, Wu S, Li F, Zhi P (2012) Adsorption of hazardous dyes indigo carmine and acid red on nanofiber membranes. RSC Adv 2:900. doi:10.1039/c1ra00546d
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem Eng J 148:354. doi:10.1016/j.cej.2008.09.009
Song W, Gao B, Xu X et al (2016) Adsorption–desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater. Bioresour Technol 210:123. doi:10.1016/j.biortech.2016.01.078
Deng J-H, Zhang X-R, Zeng G-M, Gong J-L, Niu Q-Y, Liang J (2013) Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem Eng J 226:189. doi:10.1016/j.cej.2013.04.045
Fan L, Luo C, Li X, Lu F, Qiu H, Sun M (2012) Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J Hazard Mater 215–216:272. doi:10.1016/j.jhazmat.2012.02.068
Zhao J, Ren W, Cheng H-M (2012) Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J Mater Chem 22:20197. doi:10.1039/C2JM34128J
Geng Z, Lin Y, Yu X et al (2012) Highly efficient dye adsorption and removal: a functional hybrid of reduced graphene oxide-Fe3O4 nanoparticles as an easily regenerative adsorbent. J Mater Chem 22:3527. doi:10.1039/C2JM15544C
Sui Z, Meng Q, Zhang X, Ma R, Cao B (2012) Green synthesis of carbon nanotube-graphene hybrid aerogels and their use as versatile agents for water purification. J Mater Chem 22:8767. doi:10.1039/C2JM00055E
Yu J-x, Cai X-l, Feng L-y et al (2015) Synergistic and competitive adsorption of cationic and anionic dyes on polymer modified yeast prepared at room temperature. J Taiwan Inst Chem Eng 57:98. doi:10.1016/j.jtice.2015.05.018
Yu J-x, Zhu J, Feng L-y, Chi R-a (2015) Simultaneous removal of cationic and anionic dyes by the mixed sorbent of magnetic and non-magnetic modified sugarcane bagasse. J Colloid Interface Sci 451:153. doi:10.1016/j.jcis.2015.04.009
Mahmoodi NM, Ghobadi J (2015) Extended isotherm and kinetics of binary system dye removal using carbon nanotube from wastewater. Desalin Water Treat 54:2777. doi:10.1080/19443994.2014.903525
Eftekhari S, Habibi-Yangjeh A, Sohrabnezhad S (2010) Application of AlMCM-41 for competitive adsorption of methylene blue and rhodamine B: thermodynamic and kinetic studies. J Hazard Mater 178:349. doi:10.1016/j.jhazmat.2010.01.086
Turabik M (2008) Adsorption of basic dyes from single and binary component systems onto bentonite: simultaneous analysis of Basic Red 46 and Basic Yellow 28 by first order derivative spectrophotometric analysis method. J Hazard Mater 158:52. doi:10.1016/j.jhazmat.2008.01.033
Das B, Voggu R, Rout CS, Rao CNR (2008) Changes in the electronic structure and properties of graphene induced by molecular charge-transfer. Chem Commun. doi:10.1039/b808955h
Sharma P, Hussain N, Borah DJ, Das MR (2013) Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet-water interface: a comparative study. J Chem Eng Data 58:3477. doi:10.1021/je400743r
Wu T, Cai X, Tan S, Li H, Liu J, Yang W (2011) Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem Eng J 173:144. doi:10.1016/j.cej.2011.07.050
O’Neill C, Hawkes FR, Hawkes DL, Lourenco ND, Pinheiro HM, Delee W (1999) Colour in textile effluents–sources, measurement, discharge consents and simulation: a review. J Chem Technol Biotechnol 74:1009. doi:10.1002/(sici)1097-4660(199911)74:11<1009:aid-jctb153>3.0.co;2-n
Fu YZ, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. Bioresour Technol 79:251. doi:10.1016/s0960-8524(01)00028-1
Acknowledgements
This work was supported by the National Natural Science Foundation of Zhejiang Province (Grant No. R14E030003), the National Natural Science Foundation of China (Grant Nos. 51573157, 51333004, and 51373149), and the Major Projects of Science and Technology Plan of Guizhou Province (Grant No. (2013) 6016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author declares no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, J., Lv, W., Xie, Z. et al. l-cysteine-reduced graphene oxide/poly(vinyl alcohol) ultralight aerogel as a broad-spectrum adsorbent for anionic and cationic dyes. J Mater Sci 52, 5807–5821 (2017). https://doi.org/10.1007/s10853-017-0818-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0818-y