Abstract
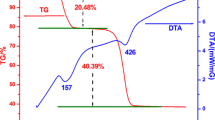
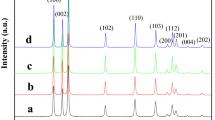
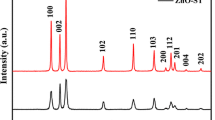
We report a simple solution-based method to synthesize phase- and size-controllable ZnS nanoparticles at low temperature. Cubic ZnS (c-ZnS) and hexagonal ZnS nanoparticles (h-ZnS) were obtained by heating an aqueous solution of Zn(NO3)2·6H2O and Na2S2O3·5H2O at different temperatures. When the system was heated at 65 °C for 24 h, hexagonal crystal structure of ZnS nanoparticles, with size of 50–350 nm, was obtained, as confirmed by X-ray diffraction and selected-area electron diffraction. When the reaction temperature was 100 °C under hydrothermal condition, c-ZnS nanoparticles were obtained and exhibited monodisperse nanoparticles with average size of 4 nm. Proper rate of S releasing tuned by the variation of pH value is believed to be critical to stabilize the hexagonal ZnS nanoparticles. Compared with large size of h-ZnS nanoparticles, c-ZnS nanoparticles show higher photocatalytic activity in degrading methyl orange (MO). The degradation efficiency of c-ZnS nanoparticles reaches 97% under UV irradiation for 120 min. The good ultraviolet absorbing ability, charge separation property, and large surface area of c-ZnS nanoparticles are believed to have a positive impact on improving the degradation rate and degradation efficiency of MO.






Similar content being viewed by others
References
Sun X, Huang X, Guo J, Zhu W, Ding Y, Niu G, Chen X (2014) Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. J Am Chem Soc 136:1706–1709
Prakasam BA, Lahtinen M, Peuronen A, Muruganandham M, Kolehmainen E, Haapaniemi E, Sillanpää M (2015) Phase selective synthesis of ZnS nanoparticles from structurally new dithiocarbamate precursor. Mater Lett 144:19–21
Wang Z, Daemen LL, Zhao Y, Zha CS, Downs RT, Wang X, Hemley RJ (2005) Morphology-tuned wurtzite-type ZnS nanobelts. Nat Mater 4:922–927
Zhang X, Yang S, Sun LQ, Luo AQ (2016) Surface-imprinted polymer coating l-cysteine-capped ZnS quantum dots for target protein specific recognition. J Mater Sci 51:6075–6085. doi:10.1007/s10853-016-9914-7
Kozák O, Praus P, Kočí K, Klementová M (2010) Preparation and characterization of ZnS nanoparticles deposited on montmorillonite. J Colloid Interface Sci 352:244–251
Gilbert B, Frazer BH, Zhang H, Huang F, Banfield JF, Haskel D, De Stasio G (2002) X-ray absorption spectroscopy of the cubic and hexagonal polytypes of zinc sulfide. Phys Rev B 66:2452053915
Fran K, Yun HN, Yiming T, Rose A, Nagarajan V, Judy NH (2016) ZnS thin films for visible-light active photoelectrodes: effect of film morphology and crystal structure. Cryst Growth Des 16:2461–2465
Fang Z, Wen S, Ye X, Feng W, Zheng Z, Lu M, Lin S, Fu X, Liu P (2015) Defect engineering and phase junction architecture of wide-bandgap ZnS for conflicting visible light activity in photocatalytic H2 evolution. ACS Appl Mater Interfaces 7:13915–13924
Geng BY, Liu XW, Du Q, Wei XW, Zhang LD (2006) Structure and optical properties of periodically twinned ZnS nanowires. Appl Phys Lett 88:163104
Tong H, Zhu YJ, Yang LX, Li L, Zhang L, Chang J, Wang SW (2007) Self-assembled ZnS nanostructured spheres: controllable crystal phase and morphology. J Phys Chem C 111:3893–3900
Zhao Y, Zhang Y, Zhu H, Hadjipanayis GC, Xiao JQ (2004) Low-temperature synthesis of hexagonal (wurtzite) ZnS nanocrystals. J Am Chem Soc 126:6874–6875
Dong M, Zhang J, Yu JG (2015) Effect of effective mass and spontaneous polarization on photocatalytic activity of wurtzite and zinc-blende ZnS. Appl Mater 3:1–8
Meng X, Xiao H, Wen X, Goddard WA III, Li S, Qin G (2013) Dependence on the structure and surface polarity of ZnS photocatalytic activities of water splitting: first-principles calculations. Phys Chem Chem Phys 15:9531–9539
Hong Y, Zhang J, Wang X, Wang Y, Lin Z, Yu J, Huang F (2012) Influence of lattice integrity and phase composition on the photocatalytic hydrogen production efficiency of ZnS nanomaterials. Nanoscale 4:2859–2862
Chen F, Cao Y, Jia D (2015) Facile synthesis of ZnS nanoparticles and their excellent photocatalytic performance. Ceram Int 41:6645–6652
Yin L, Wang D, Huang J, Cao L, Ouyang H, Yong X (2015) Morphology-controllable synthesis and enhanced photocatalytic activity of ZnS nanoparticles. J Alloys Compd 664:476–480
Ibupoto ZH, Khun K, Liu X, Willander M (2013) Hydrothermal synthesis of nanoclusters of ZnS comprised on nanowires. Nanomaterials 3:564–571
Yin L, Zhang D, Wang D, Kong X, Huang J, Wang F, Wu Y (2016) Size dependent photocatalytic activity of ZnS nanostructures prepared by a facile precipitation method. Mater Sci Eng, B 208:15–21
Smith AM, Nie SM (2010) Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc Chem Res 43:190–200
Ding Y, Wang XD, Wang ZL (2004) Phase controlled synthesis of ZnS nanobelts: zinc blende vs wurtzite. Chem Phys Lett 398:32–36
Liu W (2006) Low temperature synthesis of hexagonal phase ZnS nanocrystals by thermolysis of an air-stable single-source molecular precursor in air. Mater Lett 60:551–554
Yu SH, Yoshimura M (2002) Shape and phase control of ZnS nanocrystals: template fabrication of wurtzite ZnS single-crystal nanosheets and ZnO flake-like dendrites from a lamellar molecular precursor ZnS·(NH2CH2CH2NH2)0.5. Adv Mater 14:296–300
Huang F, Banfield JF (2005) Size-dependent phase transformation kinetics in nanocrystalline ZnS. J Am Chem Soc 127:4523–4529
Yang F, Xi J, Gan LY, Wang Y, Lu S, Ma W, Zhao Y (2016) Improved charge transfer and photoelectrochemical performance of CuI/Sb2S3/TiO2 heterostructure nanotube arrays. J Colloid Interface Sci 464:1–9
Jia Y, Yang F, Cai F, Cheng C, Zhao Y (2013) Photoelectrochemical and charge transfer properties of SnS/TiO2 heterostructure nanotube arrays. Electron Mater Lett 9:287–291
Cai FG, Yang F, Jia YF, Ke C, Cheng CH, Zhao Y (2013) Bi2S3-modified TiO2 nanotube arrays: easy fabrication of heterostructure and effective enhancement of photoelectrochemical property. J Mater Sci 48:6001–6007. doi:10.1007/s10853-013-7396-4
Cai FG, Yang F, Zhang Y, Ke C, Cheng C, Zhao Y, Yan G (2014) PbS sensitized TiO2 nanotube arrays with different sizes and filling degrees for enhancing photoelectrochemical properties. Phys Chem Chem Phys 16:23967–23974
Chai L, Du J, Xiong S, Li H, Zhu Y, Qian Y (2007) Synthesis of wurtzite ZnS nanowire bundles using a solvothermal technique. J Phys Chem C 111:12658–12662
Wang GZ, Geng BY, Huang XM, Wang YW, Li GH, Zhang LD (2003) A convenient ultrasonic irradiation technique for in situ synthesis of zinc sulfide nanocrystallites at room temperature. Appl Phys A 77:933–936
Tarasov K, Houssein D, Destarac M, Marcotte N, Gérardin C, Tichit D (2013) Stable aqueous colloids of ZnS quantum dots prepared using double hydrophilic block copolymers. New J Chem 37:508–514
Lin YH, Wang DJ, Zhao QD, Yang M, Zhang QL (2004) A study of quantum confinement properties of photogenerated charges in ZnO nanoparticles by surface photovoltage spectroscopy. J Phys Chem B 108:3202–3206
Acknowledgements
Authors acknowledge Major Project of Education Department in Sichan Province (15ZA0286), Startup Fund for Distinguished Scholars of Neijiang Normal University (14B03), Key Lab of Process Analysis and Control of Sichuan Universities (Major Project, 2015002) and Major Project of Science & Technology Department in Sichan Province (2016JY0168), New Teachers’ Fund for Doctor Stations, Ministry of Education (20120184120024) and National Magnetic Confinement Fusion Science Programme (2011GB112001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huo, F., Wang, Y., You, C. et al. Phase- and size-controllable synthesis with efficient photocatalytic activity of ZnS nanoparticles. J Mater Sci 52, 5626–5633 (2017). https://doi.org/10.1007/s10853-017-0797-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0797-z