Abstract
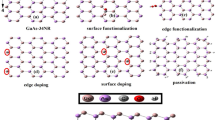
Modification of nanostructures is essential in designing materials for application in electronics and optoelectronics. In this article, the electronic structure tuning and optical properties engineering of modified graphyne (GY) and graphdiyne (GDY) are investigated by first principles density functional theory (DFT) calculations. The model GY/GDY nanoflakes are subjected to i) edge functionalization by carbonyl and carboxyl groups and ii) doping with N atom and codoping with N,S atoms. The change in the electronic and optical properties of GY/GDY due to systematic functionalization and doping is reported. It is observed that the concentration of impurity is important to tune the energy gap. The energy gap for GY/GDY flakes can be tuned over a range ~1.20 eV by varying the concentration of CO functional group. In contrast, the energy gap is insensitive to the number of COOH groups. Alternatively, the energy gap can be controlled from 0.11 to 0.68 eV by varying the N/S doping level. Upon codoping, S atom plays a role of hole doping and N acts as an electron doping. The optical response of considered systems was also monitored from the infrared to the UV region. Red shift of absorption peaks has been observed for the doped and functionalized GY/GDY flakes as compared to the original pristine systems. Increasing the dopant content results in intensive peaks which are highly shifted to the lower energies. This tunable optical response indicates that modified GY/GDY nanoflakes are prominent candidates for application in UV-light protection devices.













Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Smalley RE (1997) Discovering the fullerenes. Rev Mod Phys 69:723–730
Calizo I, Balandin AA, Bao W, Miao F, Lau CN (2007) Temperature dependence of the raman spectra of graphene and graphene multilayers. Nano Lett 7:2645–2649
Balandin AA (2011) Thermal properties of graphene and nanostructured carbon materials. Nat Mater 10:569–581
Baughman R, Eckhardt H, Kertesz J (1987) Structure property predictions for new planar forms of carbon: layered phases containing sp2 and sp atoms. J Chem Phys 87:6687–6699
Haley M, Brand C, Pak J (1997) Graphene and applications. Chem Int 36:836–838
Peng Q, Dearden AK, Crean J, Han L, Liu Sh, Wen X, De S (2017) New materials graphyne, graphdiyne, graphone, and graphane: review of properties, synthesis, and application in nanotechnology. Nanotechnol Sci Appl 7:1–29
Junjie H, Shuang Ying M, Zhou P, Zhang CX, Chaoyu H, Sun LZ (2012) Magnetic properties of single transition-metal atom absorbed graphdiyne and graphyne sheet from DFT+ U calculations. J Phys Chem C 116:26313–26321
Diederich F (1994) Carbon scaffolding: building acetylenic all-carbon and carbon-rich compound. Nature 369:199–207
Coluci VR, Braga SF, Legoas SB, Galvão DS, Baughman RH (2003) Families of carbon nanotubes: graphyne-based nanotubes. Phys Rev B 68:035430. doi:10.1103/PhysRevB.68.035430
Li G, Li Y, Liu H, Guo Y, Li Y, Zhu D (2010) Architecture of graphdiyne nanoscale films. Chem Commun 46:3256–3258
Zhang H, Zhao X, Zhang M, Luo Y, Li G, Zhao M (2013) Three-dimensional diffusion of molecular hydrogen in graphdiyne: a first-principles study. J Phys D: Appl Phys 46:495307. doi:10.1088/0022-3727/46/49/495307
Yan Z, Wang L, Cheng J, Huang L, Zhu C, Chen C, Miao L, Jiang J (2014) Lithium-decorated oxidized graphyne for hydrogen storage by first principles study. J Appl Phys 116:174304. doi:10.1063/1.4900435
Hwang HJ, Kwon Y, Lee H (2012) Thermodynamically stable calcium-decorated graphyne as a hydrogen storage medium. J Phys Chem C 116:20220–20224
Sun Ch, Searles DJ (2012) Lithium storage on graphdiyne predicted by DFT calculations. J Phys Chem C 116:26222–26226
Jang B, Koo J, Park M, Lee H, Nam J, Kwon Y, Lee H (2013) Graphdiyne as a high-capacity lithium ion battery anode material. Appl Phys Lett 103:263904. doi:10.1063/1.4850236
Lalitha M, Mahadevan SS, Lakshmipathi S (2017) Improved lithium adsorption in boron- and nitrogen substituted graphene derivatives. J Mater Sci 52:815–831. doi:10.1007/s10853-016-0378-6
Hwang HJ, Koo J, Park M, Park N, Kwon Y, Lee H (2013) Multilayer graphynes for lithium ion battery anode. J Phys Chem C 117:6919–6923
Kan EJ, Li ZY, Yang JL, Hou JG (2008) Half-metallicity in edge-modified zigzag graphene nanoribbons. J Am Chem Soc 130:4224–4225
Son YW, Cohen ML, Louie SG (2006) Energy gaps in graphene nanoribbons. Phys Rev Lett 97:216803. doi:10.1103/PhysRevLett.97.216803
Bu H, Zhao M, Zhang H, Wang X, Xi Y, Wang Z (2012) Isoelectronic doping of graphdiyne with boron and nitrogen: stable configurations and band gap modification. J Phys Chem B 116:3934–3939
Deng X, Si M, Dai J (2012) Communication: oscillated band gaps of B/N-codoped α-graphyne. J Chem Phys 137:201101. doi:10.1063/1.4769354
Zhou J, Lv K, Wang Q, Chen XS, Sun Q, Jena P (2011) Electronic structures and bonding of graphyne sheet and its BN analog. J Chem Phys 134:174701. doi:10.1063/1.3583476
Bhattacharya B, Singh NB, Sarkar U (2015) Pristine and BN doped graphyne derivatives for UV light protection. Int J Quantum Chem 115:820–829
Koo J, Huang B, Lee H, Kim G, Nam J, Kwon Y, Lee H (2014) Tailoring the electronic band gap of graphyne. J Phys Chem C 118:2463–2468
Bhattacharya B, Singh NB, Sarkar U (2014) Tuning of band gap due to fluorination of graphyne and graphdiyne. J Phys: Conf Ser 566:012014. doi:10.1088/1742-6596/566/1/012014
Long M, Tang L, Wang D, Li Y, Shuai Z (2011) Electronic structure and carrier mobility in graphdiyne Sheet and nanoribbons: theoretical predictions. ACS Nano 5:2593–2600
Wu W, Guo W, Zeng XC (2013) Intrinsic electronic and transport properties of graphyne sheets and nanoribbons. RSC Nanoscale 5:9264–9276
Kehoe JM, Kiley JH, English JJ, Johnson CA, Petersen RC, Haley M (2000) Carbon networks based on dehydrobenzoannulenes. 3. synthesis of graphyne substructures. Org Lett 2:969–972
Haley MM (2008) Synthesis and properties of annulenic subunits of graphyne and graphdiyne nanoarchitectures. Pure Appl Chem 80:519–532
Johnson CA, Lu Y, Haley MM (2007) Carbon networks based on benzocyclynes. 6. synthesis of graphyne substructures via directed alkyne metathesis. Org Lett 9:3725–3728
Yoshimura T, Inaba A, Sonoda M, Tahara K, Tobe Y, Williams RV (2006) Synthesis and properties of trefoil-shaped tris(hexadehydrotribenzo[12] annulene) and tris(tetradehydrotribenzo[12]annulene). Org Lett 8:2933–2936
Liu HB, Xu JL, Li YJ, Li YL (2010) Aggregate nanostructures of organic molecular materials. Acc Chem Res 43:1496–1508
Zhou G, Paek E, Hwang GS, Manthiram A (2015) Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat Commun 6:7760. doi:10.1038/ncomms8760
Ai W, Luo Zh, Jiang J, Zhu J, Du Zh, Fan Zh, Xie L, Zhang H, Huang W, Yu T (2014) Nitrogen and sulfur codoped graphene: multifunctional electrode materials for high-performance Li-ion batteries and oxygen reduction reaction. Adv Mater 26:6186–6192
Zhang J, Yang Z, Qiu J, Lee HW (2016) Design and synthesis of nitrogen and sulfur co-doped porous carbon via two-dimensional interlayer confinement for a high-performance anode material for lithium-ion batteries. J Mater Chem A 4:5802–5809
Huang H, Zhu J, Zhang W, Sekhar Tiwary C, Zhang J, Zhang X, Jiang Q, He H, Wu Y, Huang W, Ajayan PM, Yan Q (2016) Controllable codoping of nitrogen and sulfur in graphene for highly efficient Li-oxygen batteries and direct methanol fuel cells. Chem Mater 28:1737–1744
Mohajeri A, Shahsavar A (2016) Li-decoration on the edge oxidized graphyne and graphdiyne: a first principles study. Comput Mater Sci 115:51–59
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09, revision A.02. Gaussian, Inc, Wallingford
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Shin H, Kang S, Koo J, Lee H, Kim J, Kwon Y (2014) Cohesion energetics of carbon allotropes: quantum Monte Carlo study. J Chem Phys 140:114702. doi:10.1063/1.4867544
Zhang S, Cai Y, He H, Zhang Y, Liu R, Cao H, Wang M, Liu J, Zhang G, Li Y, Liub H, Li B (2016) Heteroatom doped graphdiyne as efficient metal-free electrocatalyst for oxygen reduction reaction in alkaline medium. J Mater Chem A 4:4738–4744
Acknowledgements
This work is supported by the Shiraz University Research Council.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohajeri, A., Shahsavar, A. Tailoring the optoelectronic properties of graphyne and graphdiyne: nitrogen/sulfur dual doping versus oxygen containing functional groups. J Mater Sci 52, 5366–5379 (2017). https://doi.org/10.1007/s10853-017-0779-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0779-1