Abstract
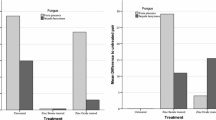
In this study, the antifungal effects of copper and silver nanoparticles against two wood-rotting fungi were investigated. European beech (Fagus sylvatica L.) and Scots pine (Pinus sylvestris L.) sapwood specimens of dimensions 50 × 25 × 15 mm3 were vacuum impregnated using dispersions of copper and silver nanoparticles within two concentrations, i.e. 1 and 3 g/l. Beech wood specimens were tested against white-rot fungus (Trametes versicolor) and pine wood against brown-rot fungus (Poria placenta) according to EN113. Furthermore, leachability, retention and protection efficiency (mass loss due to decay) were analysed afterwards. The highest value of retention was observed for pine sapwood (~2 kg/m3) for both nanoparticle solutions. The amount of nanoparticles in the wood did not increase proportionally with an increasing concentration, but only 1.5–2 times increase was reached. An average leaching of 15–35% was observed for copper nanoparticles, depending on used wood species and concentration. Significantly, lower leaching (max. 15%) was observed for pine sapwood impregnated by silver nanoparticles with a concentration of 3 g/l. The highest antifungal effect [under 5% of mass loss (ML)] against both tested fungi was found for nano-copper treatment at the concentration of 3 g/l. However, this effect of treatment seems to be almost negligible after the leaching test. Therefore, this study aims to present fundamental material properties of wood treated with copper and silver nanoparticles, and provide groundwork for further research (e.g. fixation of substances in the wood structure, etc.).






Similar content being viewed by others
References
Nel A, Xia T, Mädler L, Li N (2003) Toxic potential of materials at the nanolevel. Science 311:622–627
Civardi C, Schwarze FWMR, Wick P (2015) Micronized copper wood preservatives: an efficiency and potential health risk assessment for copper-based nano-particles. Environ Pollut 200:126–132
Kartal SN, Green IIIF, Clausen CA (2009) Do the unique properties of nanometals affect leachibility or efficiacy against fungi and termites? Int Biodeterior Biodegrad 63:490–495
Stirling R, Drummond J, Zhang J, Ziobro RJ (2008) Microdistribution of micronized copper in southern pine. International Research Group on Wood Protection, IRG/WP 08–30479
Matsunaga H, Kiguchi M, Evans PD (2009) Microdistribution of copper-carbonate and iron oxide nano-particles in treated wood. J Nanopart Res 11:1087–1098
Clausen CA (2007) Nanotechnology: implications for the wood preservation industry. International Research Group on Wood Protection. Stockholm, Sweden. IRG/WP/07-30415, pp 15
Choi OK, Hu ZQ (2009) Nitrification inhibition by silver nano-particles. Water Sci Technol 59:1699–1702
Shah V, Dobiášová P, Baldrian P, Nerud F, Kumar A, Seal S (2010) Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. J Hazard Mater 178:1141–1145
Young GY (1961) Copper tolerance of some wood-rotting fungi, Forest Products Laboratory Report 2223, pp 10
Clausen CA, Green IIIF, Woodward BM, Evans J, De Groot RC (2000) Correlation between oxalic acid production and copper tolerance in Wolfiporia cocos. Int Biodeterior Biodegrad 46:69–76
Green IIIF, Clausen CA (2005) Copper tolerance of brown-rot fungi: oxalic acid production in southern pine treated with arsenic-free preservatives. Int Biodeterior Biodegrad 56:75–79
Köse C, Kartal SN (2010) Tolerance of brown-rot and dry-rot fungi to CCA and ACQ wood preservatives. Turk J Agric For 34:181–190
Takao S (1965) Organic acid production by basidiomycetes. Appl Microbiol 13:732–737
Espejo E, Agosin E (1991) Production and degradation of oxalic acid by brown rot fungi. Appl Environ Microb 57:1980–1986
Mäkelä M, Galkin S, Hatakka A, Lundell T (2002) Production of organic acids and oxalate decarboxylase in lignin-degrading white rot fungi. Enzyme Microb Technol 30:542–549
Baldrian P (2003) Interactions of heavy metals with white rot fungi. Enzyme Microb Technol 32:78–91
Bayramoğlu G, Bektaş S, Arıca MY (2003) Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J Hazard Mater 101:285–300
Illman BI, Yang VW, Ferge L (2000) Bioprocessing preservative-treated waste wood. International Research Group on Wood Preservation, IRG/WP 00-50145, pp 11
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nano-particles. Materials 5:2850–2871
Akhtari M, Arefkhani M (2013) Study of microscopy properties of wood impregnated with nano-particles during exposed to white-rot fungus. Agric Sci Dev 2(11):116–119
Rai M, Yadav A, Gade A (2009) Silver nano-particles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Yacaman MJ (2005) The bactericidal effect of silver nano-particles. Nanotechnology 16:2346–2353
Ravishankar RV, Jamuna BA (2011) Nano-particles and their potential application as antimicrobials. In: Méndez-Vilas A (ed) Science against microbial pathogens: communicating current research and technological advances. Formatex, Badajoz
Dorau B, Arango R, Green IIIF (2004) An investigation into the potential of ionic silver as a wood preservative. In: Proceedings of the 2nd Wood-frame housing durability and disaster issues conference. Forest Products Society, Las Vegas, pp 133–145
Kim KJ, Sung WS, Suh BK, Moon SK, Choi JS, Kim JG (2009) Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 22:235–242
Pulit J, Banach M, Szczygłowska R, Bryk M (2013) Nanosilver against fungi. Silver nano-particles as an effective biocidal factor. Acta Biochim Pol 60:795–798
Rezaei VT, Usefi A, Soltani M (2011) Wood protection by nano silver against white rot. In: Proceedings of 5th Symposium on Advanceds in science and technology, Mashhad
Taghiyari HR, Schmidt O, Bari E, Tahir MDP, Karimi A, Nouri P, Jahangiri A (2014) Effect of silver nano-particles on the rate of heat transfer to the core of the medium-density fiberboard mat, IRG/WP 14-40653
Goodell B, Nicholas DD, Schultz TP (2003) Wood deterioration and preservation: advances in our changing world. American Chemical Society, Washington. ISBN 0841237972
Prucek R, Kvítek L, Panáček A, Vančurová L, Soukupová J, Jančík D, Zbořil R (2009) Polyacrylate-assisted synthesis of stable copper nano-particles and copper(I) oxide nanocubes with high catalytic efficiency. J Mater Chem 19(44):8463–8469
Kvítek L, Prucek R, Panáček A, Sivera M, Měřínská D, Tesaříková D (2015) Aqueous dispersion of silver nano-particles. Patent number 28867, Industrial Property Office (CZ)
Tascioglu C, Yalcin M, Troya TD, Sivrikaya H (2012) Termiticidal properties of some wood and bark extracts used as wood preservatives. BioResources 7(3):2960–2969
Sen S, Tascioglu C, Tirk K (2009) Fixation, leachability, and decay resistance of wood treated with some commercial extracts and wood preservative salts. Int Biodeterior Biodegrad 63:135–141
Humar M, Žlindra D, Pohleven F (2007) Influence of wood species, treatment method and biocides concentration on leaching of copper–ethanolamine preservatives. Build Environ 42:578–583
Matsunaga H, Kataoka Y, Kiguchi M, Evans P (2010) Copper nano-particles in southern pine wood treated with a micronised preservative: can nano-particles penetrate the cell walls of tracheids and ray parenchyma? IRG/WP 10–30547:14
Cooper PA, Churma R (1990) Estimating diffusion path length in treated wood. For Prod J 40:61–63
Choat B, Cobb RA, Jansen S (2007) Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol 177:608–626. doi:10.1111/j.1469-8137.2007.02317.x
Cronshow J (1960) The fine structure of the pits of (Eucalyptus regnans F.Muell.) and their relation to the movement of liquids into the wood. Aust J Bot 8:53–57
Thaler N, Humar M (2014) Copper leaching from copper-ethanolamin treated wood: comparison of field test studies and laboratory standard procedures. Bioresources 9(2):3038–3051
Richardson BA (1978) Wood preservation. E & FN SPON, London, p 1993
Lesar B, Kralj P, Žlindra D, Kancilija Humar M (2008) Comparison of standard procedures for estimation of biocides leaching from impregnated wood. Zb Gozd Lesar 86:59–64
Ding X, Meneses MB, Albukhari SM, Richter DL, Matuana LM, Heiden PA (2013) Comparing leaching of different copper oxide nanoparticles and ammoniacal copper salt from wood. Macromol Mater Eng 298:1335–1343. doi:10.1002/mame.201200439
Lebow S (1996) Leaching of wood preservative components and their mobility in the environment: summary of pertinent literature. US Department of Agriculture, Forest Service, Forest Products Laboratory, Madison, p 36
Moghaddam AH, Mulligan CN (2008) Leaching of heavy metals from chromated copper arsenate (CCA) treated wood after disposal. Waste Manag 28:628–637
Radivojevic S, Cooper PA (2010) The effects of wood species and treatment retention on kinetics of CCA-C fixation reactions. Wood Sci Technol 44:269–282
Temiz A, Alfredsen G, Yildiz UC, Gezer ED, Köse G, Akbas S, Yildiz S (2014) Leaching and decay resistance of alder and pine wood treated with copper based wood preservatives. Maderas-Ciencia y tecnologia 16:63–76
Clausen CA, Yang VW, Arango RA, Green IIIF (2009) Feasibility of nanozinc oxide as a wood preservative. Proc Am Wood Protect Assoc 105:255–260
Pohleven J, Brzin J, Vrabec L, Leonardi A, Čokl A, Štrukelj B, Kos J, Sabotič J (2011) Basidiomycete Clitocybe nebularis is rich in lectins with insecticidal activities. Appl Microbiol Biotechnol 91(4):1141–1148
Humar M, Lesar B (2008) Fungicidal properties of individual components of copper–ethanolamine-based wood preservatives. Int Biodeterior Biodegrad 62:46–50
Guillén Y, Machuca A (2008) The effect of copper on the growth of wood-rotting fungi and a blue-stain fungus. World J Microbiol Biotechnol 24:31–37
Humar M, Petrič M, Pohleven F (2001) Changes of the pH value of impregnated wood during exposure to wood-rotting fungi. Eur J Wood Wood Prod 59:288–293
Humar M, Šentjurc M, Amartey SA, Pohleven F (2005) Influence of acidification of CCB (Cu/Cr/B) impregnated wood on fungal copper tolerance. Chemosphere 58:743–749
Hastrup ACS, Green IIIF, Clausen CA, Jensen B (2005) Tolerance of Serpula lacrymans to copper-based wood preservatives. Int Biodeterior Biodegrad 56:173–177
Gadd G (1999) Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv Microbial Physiol 11:47–91
Golubovich VN, Rabotnova IL (1974) Kinetics of growth inhibition by silver ions. Microbiology 43:948–950
Highley TL (1975) Inhibition of cellulases of wood-decay fungi. US Department of Agriculture, Forest Service, Forest Service Laboratory, Madison, p 8
Guggenbichler JP, Böswald M, Lugauer S, Krall T (1999) A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection 27:s16–s23
Vršanská M, Burešová A, Damborský P, Adam V (2015) Influence of different inducers on ligninolytic enzyme activities. J Metallomics Nanotechnol 3:64–70
Hrastnik D, Budija F, Humar M, Petrič M (2013) Influence of liquefied and CCB containing liquefied wood on growth of wood decay fungi. Maderas-Cienc Tecnol 15:105–118
Wazny J, Thornton JD (1986) Comparative laboratory testing of strains of the dry rot fungus Serpula lacrymans. Holzforschung 40:383–388
Acknowledgements
This work was carried out at Research Center Josef Ressel in Brno-Útěchov. It was financially supported by the Internal Grant Agency (IGA) of the Faculty of Forestry and Wood Technology, Mendel University in Brno, (LDF_PSV_2016015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pařil, P., Baar, J., Čermák, P. et al. Antifungal effects of copper and silver nanoparticles against white and brown-rot fungi. J Mater Sci 52, 2720–2729 (2017). https://doi.org/10.1007/s10853-016-0565-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0565-5