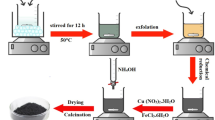
Abstract
Ultrafast rechargeable Li-ion batteries are most urgently needed for personal electronics and commercial application since they could fuel various energy applications. These days, much time and efforts have been paid on the research of ultrafast rechargeable Li-ion batteries, however, less breakthrough was appeared. In this manuscript, the CuCr2O4@RGO, a new-type of nanocomposite material, demonstrates a promising future application when it was used as an anode material of the Li-ion batteries. The Li-ion batteries displays a specific capacity of about 410 mAh g−1 and a coulombic efficiency of approximately 98 % at the discharge/charge current rate of 1000 mA g−1. We also found that the batteries constructed by CuCr2O4@RGO nanocomposites as active anode materials can be fully charged within 5 min, with a current density of 4000 mA g−1 and strive against more than 900 cycles without capacity decay. All the results indicate that the CuCr2O4@RGO nanocomposites with spinel structure of the mixed transitional metal oxide are promising for the anode materials of ultrafast rechargeable Li-ion batteries.






Similar content being viewed by others
References
Wang ZL, Xu D, Xu JJ, Zhang XB (2014) Oxygen electrocatalysts in metal–air batteries: from aqueous to nonaqueous electrolytes. Chem Soc Rev 43:7746–7786
Yang Z, Zhang J, Kintner-Meyer MCW, Lu X, Choi D, Lemmon JP, Liu J (2011) Electrochemical energy storage for green grid. Chem Rev 111:3577–3613
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Armand M, Tarascon JM (2008) Issues and challenges facing rechargeable lithium batteries. Nature 451:652–657
Aricò AS, Bruce P, Scrosati B, Tarascon J-M, van Schalkwijk W (2005) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4:366–377
Yue GH, Zhang XQ, Zhao YC, Xie QS, Zhang XX, Peng DL (2014) High performance of Ge@ C nanocables as the anode for lithium ion batteries. RSC Adv 4:21450–21455
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2000) Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407:496–499
Reddy ALM, Gowda SR, Shaijumon MM, Ajayan PM (2012) Hybrid nanostructures for energy storage applications. Adv Mater 24:5045–5064
Yue GH, Zhao YC, Wang CG, Zhang XX, Zhang XQ, Xie QS (2015) Flower-like nickel oxide nanocomposites anode materials for excellent performance lithium-ion batteries. Electrochim Acta 152:315–322
Wang JJ, Li YL, Sun XL (2013) Challenges and opportunities of nanostructured materials for aprotic rechargeable lithium–air batteries. Nano Energy 2:443–467
Goodenough JB, Park K-S (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135:1167–1176
Yuan C, Wu Hao B, Xie Y, Lou XW (2014) Mixed transition-metal oxides: design, synthesis, and energy-related applications. Angew Chem Int Ed 53:1488–1504
Kang K, Ceder G (2006) Factors that affect Li mobility in layered lithium transition metal oxides. Phys Rev B 74:094105
Wang Q, Wang X, Xu J, Xia O, Hou X, Chen D, Wang R, Shen G (2014) Flexible coaxial-type fiber supercapacitor based on NiCo2O4 nanosheets electrodes. Nano Energy 8:44–51
Zhou D, Su X, Boese M, Wang R, Zhang H (2014) Ni (OH)2@Co (OH)2 hollow nanohexagons: controllable synthesis, facet-selected competitive growth and capacitance property. Nano Energy 5:52–59
He P, Yu H, Li D, Zhou H (2012) Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J Mater Chem 22:3680–3695
Lavela P, Tirado JL (2007) CoFe2O4 and NiFe2O4 synthesized by sol–gel procedures for their use as anode materials for Li ion batteries. J Power Sour 172:379–387
Yuan W, Liu X, Li L (2014) Synthesis, characterization and photocatalytic activity of cubic-like CuCr2O4 for dye degradation under visible light irradiation. Appl Surf Sci 319:350–357
Acharyya SS, Ghosh S, Tiwari R, Pendem C, Sasaki T, Bal R (2015) Synergistic Effect between ultrasmall Cu (II) oxide and CuCr2O4 spinel nanoparticles in selective hydroxylation of benzene to phenol with air as oxidant. ACS Catal 5:2850–2858
Yan J, Zhang L, Yang H, Tang Y, Lu Zh, Guo S, Dai Y, Han Y, Yao M (2009) CuCr2O4/TiO2 heterojunction for photocatalytic H 2 evolution under simulated sunlight irradiation. J Sol Energy 83:1534–1539
Bajaj R, Sharma M, Bahadur D (2013) Visible light-driven novel nanocomposite (BiVO4/CuCr2O4) for efficient degradation of organic dye. Dalton Trans 42:6736–6744
Pan L, Li L, Bao X, Chen Y (2012) Highly photocatalytic activity for p-nitrophenol degradation with spinel-structured CuCr2O4. Micro Nano Lett 5:415–418
Beshkar F, Zinatloo-Ajabshir S, Salavati-Niasari M (2015) Preparation and characterization of the CuCr2O4 nanostructures via a new simple route. J Mater Sci 26:5043–5051
Lao M, Shun J, Shao L, Lin X, Wu K, Shui M, Li P, Long N, Ren Y (2014) Enhanced electrochemical performance of Ag-coated CuCr2O4 as anode material for lithium-ion batteries. Ceram Int 40:11899–11904
Ferrari AC, Meyer JC, Scardaci V et al (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97(18):187401
Niu Z, Liu L, Zhang L et al (2014) A universal strategy to prepare functional porous graphene hybrid architectures. Adv Mater 26:3681–3687
De S, Mandal S (2013) Surfactant-assisted shape control of copper nanostructures. Colloids Surf A 421:72–83
Hashempour M, Razavizadeh H, Rezaie H, Hashempour M, Ardestani M (2010) Chemical mechanism of precipitate formation and pH effect on the morphology and thermochemical co-precipitation of W–Cu nanocomposite powders. Mater Chem Phys 123:83–90
Heaton BT, Jacob C, Page P (1996) Transition metal complexes containing hydrazine and substituted hydrazines. Coordin Chem Rev 154:193–229
Pei S, Cheng HM (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Acharyya SS, Ghosh S, Bal R (2014) Fabrication of three-dimensional (3D) raspberry-like copper chromite spinel catalyst in a facile hydrothermal route and its activity in selective hydroxylation of benzene to phenol. ACS Appl Mater Interface 6:14451–14459
Tao HC, Fan LZ, Mei Y et al (2011) Self-supporting Si/reduced graphene oxide nanocomposite films as anode for lithium ion batteries. Electrochem Commun 13:1332–1335
Xiao L, Sehlleier YH, Dobrowolny S et al (2015) Si-CNT/rGO Nanoheterostructures as high-performance lithium-ion-battery Anodes. Chem Electro Chem 2:1983–1990
Chang J, Huang X, Zhou G et al (2014) Multilayered Si nanoparticle/reduced graphene oxide hybrid as a high-performance lithium-ion battery anode. Adv Mater 26:758–764
Chen J, Xia XH, Tu JP, Xiong QQ, Yu YX, Wang XL (2012) Co3O4-C core–shell nanowire array as an advanced anode material for lithium ion batteries. J Mater Chem 22:15056–15061
Guo B, Chi M, Sun XG, Dai S (2012) Mesoporous carbon–Cr2O3 composite as an anode material for lithium ion batteries. J Power Sour 205:495–499
Wang T, Xie G, Zhu J et al (2015) Elastic reduced graphene oxide nanosheets embedded in germanium nanofiber matrix as anode material for high-performance Li-ion battery. Electrochim Acta 186:64–70
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2001) Searching for new anode materials for the Li-ion technology: time to deviate from the usual path. J Power Sour 97:235–239
Wang SQ, Zhang JY, Chen CH (2007) Dandelion-like hollow microspheres of CuO as anode material for lithium-ion batteries. Scripta Mater 57:337–340
Xie Q, Ma Y, Zhang X, Guo H, Lu A, Wang L, Yue G, Peng D (2014) Synthesis of amorphous ZnSnO3-C hollow microcubes as advanced anode materials for lithium ion batteries. Electrochim Acta 141:374–383
Bijelić M, Liu X, Sun Q et al (2015) Long cycle life of CoMn2O4 lithium ion battery anodes with high crystallinity. J Mater Chem A 3:14759–14767
Chen H, Wang C, Dong W et al (2014) Monodispersed sulfur nanoparticles for lithium–sulfur batteries with theoretical performance. Nano Lett 15:798–802
Zhu XD, Tian J, Le SR, Zhang NQ, Sun KN (2013) Improved electrochemical performance of CuCrO2 anode with CNTs as conductive agent for lithium ion batteries. Mater Lett 97:113–116
Acknowledgements
This work was jointly supported by the National Science Foundation of China (Grant Nos. 11572271, 51171158, 51371154, and 11405144) and the National Basic Research Program of China (Grant No. 2012CB933103).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, C.G., Liu, J.D., Li, X. et al. Graphene-modified copper chromate as the anode of ultrafast rechargeable Li-ion batteries. J Mater Sci 52, 2131–2141 (2017). https://doi.org/10.1007/s10853-016-0501-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0501-8