Abstract
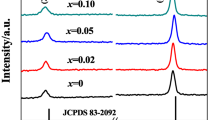
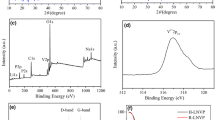
Microwave-assisted synthesis of electrode materials for lithium-ion batteries has drawn extensive attention owing to the unique microwave dielectric heating. In this work, olivine LiFePO4 hexagonal nanoplates, with a short b-axis, were successfully synthesized using a single-mode microwave-assisted hydrothermal system at 160 °C just in 20 min. Microwave irradiation can lower the synthesis temperature and shorten the synthesis time dramatically. The growth process of LiFePO4 hexagonal nanoplates with microwave irradiation time was investigated. The role of electromagnetic field in the formation and the quality of the resulting LiFePO4 were explored. In order to enhance the electrochemical properties of LiFePO4 hexagonal nanoplates, LiFePO4/C and LiFePO4/rGO have been obtained through surface decoration of LiFePO4 nanoplates by ex situ carbon coating and in situ reduced graphene oxide (rGO) coating. The electrochemical analysis demonstrated that LiFePO4/rGO had more excellent electrochemical performance; the initial discharge capacity at 0.1 C was up to 167.2 mAh g−1 which was very close to the theoretical value (170 mAh g−1). This is because the in situ coating can achieve a complete coating of the surface and rGO has a higher electrical conductivity. The rGO layer can boost the transport speed of the lithium ions and electrons, and reduce the charge transfer resistance of Li ion insertion/extraction. Furthermore, the unique structure of the nanoplates with a short b-axis is favored to shorten the migration of Li+ ion.










Similar content being viewed by others
References
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271–4301
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Mapping of transition metal redox energies in phosphates with NASICON structure by lithium intercalation. J Electrochem Soc 144:2581–2586
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Yamada A, Chung SC, Hinokuma K (2001) Optimized LiFePO4 for lithium battery cathodes. J Electrochem Soc 3:A224–A229
Dathar GKP, Sheppard D, Stevenson KJ, Henkelman G (2011) Calculations of Li-ion diffusion in olivine phosphates. Chem Mater 23:4032–4037
Huang XJ, Yan SJ, Zhao HY, Zhang L, Guo R, Chang CK, Kong XY, Han HB (2010) Electrochemical performance of LiFePO4 nanorods obtained from hydrothermal process. Mater Charact 61:720–725
Muraliganth T, Stroukoff KR, Manthiram A (2010) Microwave-solvothermal synthesis of nanostructured Li2MSiO4/C (M=Mn and Fe) cathodes for lithium-ion batteries. Chem Mater 22:5754–5761
Malik R, Burch D, Bazantand M, Ceder G (2010) Particle size dependence of the ionic diffusivity. Nano Lett 10:4123–4127
Ding Y, Jiang Y, Xu F, Yin J, Ren H, Zhuo Q, Long Z, Zhang P (2010) Preparation of nano-structured LiFePO4/graphene composites by co-precipitation method. Electrochem Commun 12:10–13
Zhang Y, Du PP, Wang LZ, Zhang AQ, Song YH, Li XF, Lv Y (2011) Synthesis and electrochemical properties of gyroscope-like lithium iron phosphate/multiwalled carbon nanotubes composites by microwave-assisted sol–gel method. Synth Met 161:548–551
Niu B, Qi EL, Wang JQ (2011) A simple and facile preparation of LiFePO4 by a one-step microwave hydrothermal method. J Inorg Organomet Polym Mater 21:906–912
Wang SP, Yang HX, Feng LJ, Sun SM, Guo JX, Yang YZ, Wei HY (2013) A simple and inexpensive synthesis route for LiFePO4/C nanoparticles. J Power Sources 233:43–46
Ma ZP, Shao GJ, Fan YQ, Wang GL, Song JJ, Liu TT (2014) Tunable morphology synthesis of LiFePO4 nanoparticles as cathode. ACS Appl Mater Interface 6:9236–9244
Zeng GB, Caputo R, Carriazo D, Luo L, Niederberger M (2013) Tailoring two polymorphs of LiFePO4 by efficient microwave-assisted synthesis: a combined experimental and theoretical study. Chem Mater 25:3399–3407
Zhou XF, Wang F, Zhu YM, Liu ZP (2011) Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J Mater Chem 21:3353–3358
Tang YF, Huang FQ, Bi H, Liu ZQ, Wan DY (2012) Highly conductive three-dimensional graphene for enhancing the rate performance of LiFePO4 cathode. J Power Sources 203:130–134
Kang FY, Mi J, Li BH (2011) Effects of carbonaceous materials on the physical and electrochemical performance of a LiFePO4 cathode for lithium-ion batteries. New Carbon Mater 26:161–170
Shi Y, Chou SL, Wang JZ, Wexler D, Li HJ, Liu H, Wu YP (2012) Graphene wrapped LiFePO4/C composites as cathode materials for Li-ion batteries with enhanced rate capability. J Mater Chem 22:16465–16470
Gao HY, Jiao LF, Peng WX, Liu G, Yang JQ, Zhao QQ, Qi Z, Si YC, Wang YJ, Yuan HT (2011) Enhanced electrochemical performance of LiFePO4/C via Mo-doping at Fe site. Electrochim Acta 56:9961–9967
Yang JM, Bai Y, Qing CB, Zhang WF (2011) Electrochemical performances of Co-doped LiFePO4/C obtained by hydrothermal method. J Alloy Compd 509:9010–9014
Wang GX, Shen XP, Yao J (2009) One-dimensional nanostructures as electrode materials for lithium-ion batteries with improved electrochemical performance. J Power Sources 189:543–546
Sabina B, Libero D, Marina M (2009) Fast sol–gel synthesis of LiFePO4/C for high power lithium-ion batteries for hybrid electric vehicle application. J Power Sources 194:1094–1098
Kiyoshi K, Shohei K, Kaoru D (2008) Hydrothermal synthesis of LiFePO4 as a cathode material for lithium batteries. J Mater Sci 43:2138–2142. doi:10.1007/s10853-007-2011-1
Li ZH, Zhang DM, Yang FX (2009) Developments of lithium-ion batteries and challenges of LiFePO4 as one promising cathode material. J Mater Sci 44:2435–2443. doi:10.1007/s10853-009-3316-z
Xiang HF, Chen CH, Zhang DW, Wu JS, Jin Y, Wang HH (2011) Hydrothermal synthesis of ultra-thin LiFePO4 platelets for Li-ion batteries. J Mater Sci 46:4906–4912. doi:10.1007/s10853-011-5403-1
Nagaraju DH, Mirjana K, Suresh GS (2015) LiFePO4 wrapped reduced graphene oxide for high performance Li-ion battery electrode. J Mater Sci 50:4244–4249. doi:10.1007/s10853-015-8976-2
Chang YC, Hung IM, Peng CT (2014) Effects of particle size and carbon coating on electrochemical properties of LiFePO4/C prepared by hydrothermal method. J Mater Sci 49:6907–6916. doi:10.1007/s10853-014-8395-9
Zhou XW, Zhan D, Cong CJ, Guo GH, Wang LN (2005) Synthesis and electrochemical properties of LiFePO4/C cathode material. J Mater Sci 40:2577–2578. doi:10.1007/s10008-011-1426-4
Xiao PF, Lai MO, Lu L (2013) Hollow microspherical LiFePO4/C synthesized from a novel multidentate phosphonate complexing agent. RSC Adv 3:5127–5130
Qiu YJ, Zuo XB, Geng YH, Yu J (2014) High-capacity cathode for lithium-ion battery from LiFePO4/(C+Fe2P) composite nanofibers by electrospinning. J Mater Sci 49:504–509. doi:10.1007/s10853-013-7727-5
Zhang L, Xiang HF, Yang WS, Zhu XF, Wang HH (2012) Synthesis of LiFePO4/C composite as a cathode material for lithium-ion battery by a novel two-step method. J Mater Sci 47:3076–3081. doi:10.1007/s10853-011-6139-7
Li YD, Zhao SX, Nan CW, Li BH (2011) Electrochemical performance of SiO2-coated LiFePO4 cathode materials for lithium ion battery. J Alloy Compd 509:957–960
Deng HG, Jin SL, Zhan L, Qiao WM, Ling LC (2012) Nest-like LiFePO4/C architectures for high performance lithium ion batteries. Electrochim Acta 78:633–637
Deng HG, Jin SL, Zhan L, Wang YL, Qiao WM, Ling LC (2012) Synthesis of cage-like LiFePO4/C microspheres for high performance lithium ion batteries. J Power Sources 220:342–347
Zhou N, Wang HY, Chaker EU, Zhang M, Liu SQ, Liu YN, Cao GZ (2013) Additive-free solvothermal synthesis and Li-ion intercalation properties of dumbbell-shaped LiFePO4/C mesocrystals. J Power Sources 239:103–110
Zhou N, Uchaker E, Liu N, Wang HY, Zhang M, Liu SQ, Liu YN, Wu XW, Cao GZ, Li HY (2013) Additive-free solvothermal synthesis of hierarchical flower-like LiFePO4/C mesocrystal and its electrochemical performance. RSC Adv 3:19366–19374
Wang Q, Deng SX, Wang H, Xie M, Liu JB, Yan H (2013) Hydrothermal synthesis of hierarchical LiFePO4 microspheres for lithium ion battery. J Alloy Compd 553:69–74
Cho MY, Kim KB, Lee JW, Kim H, Kim H, Kang K, Roh KC (2013) Defect-free solvothermally assisted synthesis of microspherical mesoporous LiFePO4/C. RSC Adv 3:3421–3427
Nina IK, Patricia JO, Benjamin RM, Thomas EM, Sergey AC, Eugenia VB, Alexandr DG (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11:771–778
Wang L, Wang HB, Liu ZH, Xiao C, Dong SM, Han PX, Zhang ZY, Zhang XY, Bi CF, Cui GL (2010) A facile method of preparing mixed conducting LiFePO4/graphene composites for lithium-ion batteries. Solid State Ion 181:1685–1689
Burba CM, Frech R (2004) Raman and FTIR spectroscopic study of LixFePO4 (0 ≤ x ≤ 1). J Electrochem Soc 151:A1032–A1038
Wu J, Dathar GKP, Sun CW, Theivanayagam MG, Applestone D, Dylla AG, Manthiram A, Henkelman G, Goodenough JB, Stevenson KJ (2013) In situ Raman spectroscopy of LiFePO4: size and morphology dependence during charge and self-discharge. Nanotechnology 24:1–9
Wang GX, Shen XP, Yao J, Park J (2009) Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 47:2049–2053
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia YY, Wu Y, Nguyen SBT, Ruof RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Doeff MM, Hu YQ, McLarnon F, Kostecki R (2003) Effect of surface carbon structure on the electrochemical performance of LiFePO4. Electrochem Solid State 6(10):A207–A209
Kostecki R, Schnyder B, Alliata D, Song X, Kinoshita K, Kotz R (2001) Surface studies of carbon films from pyrolyzed photoresist. Thin Solid Films 396:36–43
Tuinstra F, Koenig JL (1970) Raman Spectrum of graphite. J Chem Phys 53:1126–1130
Xu HR, Gao L (2002) New evidence of a dissolution–precipitation mechanism in hydrothermal synthesis of barium titanate powders. Mater Lett 57:490–494
Qin X, Wang XH, Xiang HM, Xie J, Li JJ, Zhou YC (2010) Mechanism for hydrothermal synthesis of LiFePO4 platelets as cathode material for lithium-ion batteries. J Phys Chem C 114:16806–16812
Qian JF, Zhou M, Cao YL, Ai XP, Yang HX (2010) Template-free hydrothermal synthesis of nanoembossed mesoporous LiFePO4 microspheres for high-performance lithium-ion batteries. J Phys Chem C 114:3477–3482
Li J, Qu Q, Zhang LF, Zhang L, Zheng HH (2013) A monodispersed nano-hexahedral LiFePO4 with improved power capability by carbon-coatings. J Alloy Compd 579:377–383
Ni JF, Zhou HH, Chen JT, Zhang XX (2005) LiFePO4 doped with ions prepared by co-precipitation method. Mater Lett 59:2361–2365
Murugan AV, Muraliganth T, Manthiram A (2009) One-pot microwave-hydrothermal synthesis and characterization of carbon-coated LiMPO4 (M=Mn, Fe, and Co) cathodes. J Electrochem Soc 156:A79–A83
Su FY, He YB, Li BH, Chen XC, You CH, Lv WW, Yang QH, Kang FY (2012) Could graphene construct an effective conducting network in a high-power lithium ion battery? Nano Energy 1:429–439
Acknowledgements
This work was supported by the Chinese National Science Foundation (51172175, 51072147) and the Hubei Science & Technology Plan (2012FFB05107, 2013BKB006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, C., Zhou, J., Liu, G. et al. Microwave-assisted synthesis and surface decoration of LiFePO4 hexagonal nanoplates for lithium-ion batteries with excellent electrochemical performance. J Mater Sci 52, 1590–1602 (2017). https://doi.org/10.1007/s10853-016-0453-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0453-z