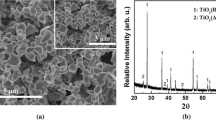
Abstract
Titanium nitride powders were synthesized from titanium dioxide at 1173–1373 K in ammonia atmosphere. The reduction–nitridation products with various fractions obtained at various temperatures were analyzed by X-ray diffraction, Raman spectra, X-ray photoelectron spectroscopy, scanning electron microscope, transmission electron microscopy, and selected area electron diffraction. The reaction sequence from TiO2 to TiN in ammonia atmosphere was changed by increasing the reaction temperature. The reaction sequence at 1173 K was found as TiO2 → TiN1−xOx → TiN. When the reaction temperature was above 1273 K, the reaction sequence changed to as follows: TiO2 → Ti9O17 → TiN1−xOx → TiN. Ti3O5 was not found as an intermediate phase on account of its instability in NH3 atmosphere. The morphology of the synthesized TiN is closely related to that of the raw materials.















Similar content being viewed by others
References
Zhang HJ, Li FL, Jia QL (2009) Preparation of titanium nitride ultrafine powders by sol–gel and microwave carbothermal reduction nitridation methods. Ceram Int 35:1071–1075
Guo HJ, Chen WY, Shan Y, Wang WZ, Zhang ZY, Jia JH (2015) Microstructures and properties of titanium nitride films prepared by pulsed laser deposition at different substrate temperatures. Appl Surf Sci 357:473–478
Ru JJ, Hua YX, Xu CY, Zhang QB, Wang D, Gong K (2014) Synthesis of TiN from FeTiO3 by microwave-assisted carbothermic reduction–nitridation. J Alloys Compd 583:121–127
Grigorov GI, Grigorov KG, Stoyanova M, Vignes JL, Langeron JP, Denjean P (1993) Aluminium diffusion in titanium nitride films. Efficiency of TiN barrier layers. Appl Phys A 57:195–197
Mosavati N, Chitturi VR, Salley SO, Ng KS (2016) Nanostructured titanium nitride as a novel cathode for high performance lithium/dissolved polysulfied batteries. J Power Sources 321:87–93
Zega B, Kornmann M, Amiguet J (1997) Hard decorative TiN coatings by ion plating. Thin Solid Films 45:577–582
Skopp A, Woydt M (1995) Ceramic and ceramic composite materials with improved friction and wear properties. Tribol Trans 38:233–242
Aghababazadeh R, Mirhabibi AR, Rand B, Banijamali S, Pourasad J, Ghahari M (2007) Synthesis and characterization of nanocrystalline titanium nitride powder from rutile and anatase as precursors. Surf Sci 601:2881–2885
White GV, Mackenzie KJ, Johnston JH (1992) Carbothermal synthesis of titanium nitride. J Mater Sci 27:4287–4293. doi:10.1007/BF00541554
Jha A, Yoon SJ (1999) Formation of titanium carbonitride phases via the reduction of TiO2 with carbon in the presence of nitrogen. J Mater Sci 34:307–322. doi:10.1023/A:1004457622751
Eslamloo-Grami M, Munir ZA (1990) Effect of nitrogen pressure and diluent content on the combustion synthesis of titanium nitride. J Am Ceram Soc 73:2222–2227
Kim W, Park JS, Suh CY, Cho SW, Lee S, Shon IJ (2009) Synthesis of TiN nanoparticles by explosion of Ti wire in nitrogen gas. Mater Trans 50:2897–2899
Dekker JP, Put PJ, Ceringa HJ, Schoonman J (1994) Vapour-phase synthesis of titanium nitride powder. J Mater Chem 4:689–694
Zhang F, Kaczmarek WA, Lu L, Lai MO (2000) Formation of titanium nitrides via wet reaction ball milling. J Alloys Compd 307:249–253
Bolokang AS, Phasha MJ (2010) Formation of titanium nitride produced from nanocrystalline titanium powder under nitrogen atmosphere. Int J Refract Metals Hard Mater 28:610–615
Joshi UA, Chung SH, Lee JS (2005) Low-temperature, solvent-free solid-state synthesis of single-crystalline titanium nitride nanorods with different aspect ratios. J Solid State Chem 178:755–760
Li WY, Riley FL (1991) The production of titanium nitride by the carbothermal nitridation of titanium dioxide powder. J Eur Ceram Soc 8:345–354
Peelamedu RD, Fleming M, Agrawal DK, Roy R (2002) Preparation of titanium nitride: microwave-induced carbothermal reaction of titanium dioxide. J Am Ceram Soc 85:117–122
Ahn YU, Kim EJ, Kim HT, Hahn SH (2003) Variation of structural and optical properties of sol-gel TiO2 thin films with catalyst concentration and calcination temperature. Mater Lett 57:4660–4666
Arsov LK, Kormann C, Plieth W (1991) Electrochemical synthesis and in situ Raman spectroscopy of thin films of titanium dioxide. J Raman Spectrosc 22:573–575
Zhang Z, Goodall JB, Morgan DJ, Brown S, Clark RJ, Knowles JC, Mordan NJ, Evans JR, Carley AF, Bowder M, Dan JA (2009) Photocatalytic activities of N-doped nano-titanias and titanium nitride. J Eur Ceram Soc 29:2343–2353
Drygas M, Czosnek C, Paine RT, Janik JF (2006) Two-stage aerosol synthesis of titanium nitride TiN and titanium oxynitride TiOxNy nanopowders of spherical particle morphology. Chem Mater 18:3122–3129
Peng F, Cai LF, Huang L, Yu H, Wang HJ (2008) Preparation of nitrogen-doped titanium dioxide with visible-light photocatalytic activity using a facile hydrothermal method. J Phys Chem Solids 69:1657–1664
Trenczek-Zajac A, Radecka M, Zakrzewska K, Brundnik A, Kusior E, Bourgeois S, Marco de Lucas MC, Imhoff L (2009) Structural and electrical properties of magnetron sputtered Ti(ON) thin film: the case of TiN doped in situ with oxygen. J Power Sources 194:93–103
Saha NC, Tompkins HG (1992) Titanium nitride oxidation chemistry: an X-ray photoelectron spectroscopy study. J Appl Phys 72:3072–3079
Acknowledgements
We acknowledge the financial support provided by the Fundamental Research Funds for the Central Universities (FRF-TP-15-009A3) and the Natural Science Foundation of China (No. U1460201).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gou, HP., Zhang, GH. & Chou, KC. Phase evolution and reaction mechanism during reduction–nitridation process of titanium dioxide with ammonia. J Mater Sci 52, 1255–1264 (2017). https://doi.org/10.1007/s10853-016-0420-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0420-8