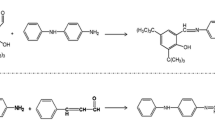
Abstract
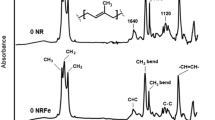
Mixed antioxidants composed of antioxidant IPPD and novel rare earth lanthanum complex were used as an additive to prepare natural rubber (NR) samples. The variations of macro-properties, surface characterizations, and internal groups were investigated by mechanical testing, X-ray photoelectron spectroscopy, and thermogravimetric analysis coupled with Fourier transform infrared spectroscopy (TGA/FT-IR), respectively, to study the thermal-oxidative stability of NR. The thermal-oxidative degradation kinetic parameters were determined by analyzing the thermogravimetric curves at different heating rates with two model-free methods, Kissinger method and Flynn–Wall–Ozawa method. The results all showed that, compared with pure antioxidant IPPD, the same mass of mixed antioxidants could indeed improve the thermal-oxidative stability of NR. Furthermore, based on the TGA/FT-IR results and quantum mechanics simulations, the autocatalytic, free radical chain reaction mechanism for the thermal-oxidative aging of NR was clarified, and the different function mechanisms of antioxidants IPPD and p-ASALa were also discussed. Except for functioning as a labile-hydrogen donor which is similar to antioxidant IPPD in protecting NR against autoxidation, p-ASALa has strong coordination abilities and large coordination numbers, resulting in the high efficiency in enhancing the thermal-oxidative stability of NR.














Similar content being viewed by others
References
Mita I, Jellinek HHG (1987) Aspects of degradation and stabilization of polymers. Elsevier, New York.
Ramesan MT, Kumar TM, Alex R, Kuriakose B (2002) Investigations on the addition of styrene butadiene rubber in natural rubber and dichlorocarbene modified styrene butadiene rubber blends. J Mater Sci 37:109–116. doi:10.1023/A:1013106227399
Komethi M, Othman N, Ismail H, Sasidharan S (2012) Comparative study on natural antioxidant as an aging retardant for natural rubber vulcanizates. J Appl Polym Sci 124:1490–1500.
Wu W, Zeng X, Li H, Lai X, Li F, Guo J (2014) Synthesis and characterization of a novel macromolecular hindered phenol antioxidant and its thermo-oxidative aging resistance for natural rubber. J Macromol Sci B 53:1244–1257.
Zheng W, Liu L, Zhao X, He J, Wang A, Chan TW, Wu S (2015) Effects of lanthanum complex on the thermo-oxidative aging of natural rubber. Polym Degrad Stab 120:377–383.
Luo Y, Yang C, Chen B, Xu K, Zhong J, Peng Z, Lv Z, Wang Q (2013) Thermal degradation of epoxidized natural rubber in presence of neodymium stearate. J Rare Earths 31:526–530.
Fang L, Song Y, Zhu X, Zheng Q (2009) Influence of lanthanum stearate as a co-stabilizer on stabilization efficiency of calcium/zinc stabilizers to polyvinyl chloride. Polym Degrad Stab 94:845–850.
Qiu GM, Zhang M, Zhou LX, Nakakita SS, Inoue SI, Okamoto H (2001) Thermal oxidation resistance of rare earth-containing composite elastomer. J Rare Earths 19:192–197.
Xie C, Jia Z, Luo Y, Jia D (2011) Antioxidant effect of Sm(III) complex with 2-mercaptobenzimidazole in natural rubber vulcanizates. Acta Polym Sin. doi:10.3724/SP.J.1105.2011.10090.
Xie C, Jia Z, Jia D, Luo Y, You C (2010) The effect of Dy(III) complex with 2-mercaptobenzimidazole on the thermo-oxidation aging behavior of natural rubber vulcanizates. Int J Polym Mater 59:663–679.
Shelton JR (1957) Aging and oxidation of elastomers. Rubber Chem Technol 30:1251–1290.
ISO 188 (2011) Rubber vulcanized or thermoplastic—accelerated ageing and heat resistance tests. ISO Copyright Office, Switzerland.
ISO 37 (2011) Rubber vulcanized or thermoplastic—determination of tensile stress–strain properties. ISO Copyright Office, Switzerland.
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868.
Sutton CC, Franks GV, da Silva G (2012) First principles pKa calculations on carboxylic acids using the SMD solvation model: effect of thermodynamic cycle model chemistry and explicit solvent molecules. J Phys Chem B 116:11999–12006.
Ho J (2015) Are thermodynamic cycles necessary for continuum solvent calculation of pKas and reduction potentials? Phys Chem Chem Phys 17:2859–2868.
McGrath MJ, Kuo IF, Ngouana WB, Ghogomu JN, Mundy CJ, Marenich AV, Cramer CJ, Truhlar DG, Siepmann JI (2013) Calculation of the Gibbs free energy of solvation and dissociation of HCl in water via Monte Carlo simulations and continuum solvation models. Phys Chem Chem Phys 15:13578–13585.
Zhao F, Zhao S, Weina B, Kuhn W, Jian Y (2007) Characterization of elastomer networks by NMR parameters: part I. Sulfur-cured NR networks. Kautsch Gummi Kunstst (KGK) 60:554–558.
Anca-Couce A, Zobel N, Berger A, Behrendt F (2012) Smouldering of pine wood: kinetics and reaction heats. Combust Flame 159:1708–1719.
Ferriol M, Gentilhomme A, Cochez M, Oget N, Mieloszynski JL (2003) Thermal degradation of poly (methyl methacrylate) (PMMA): modelling of DTG and TG curves. Polym Degrad Stab 79:271–281.
Li KY, Huang X, Fleischmann C, Rein G, Ji J (2014) Pyrolysis of medium-density fiberboard: optimized search for kinetics scheme and parameters via a genetic algorithm driven by Kissinger’s method. Energy Fuel 28:6130–6139.
Chen M, Ao NJ, Zhang BL, Den CM, Qian HL, Zhou HL (2005) Comparison and evaluation of the thermooxidative stability of medical natural rubber latex products prepared with a sulfur vulcanization system and a peroxide vulcanization system. J Appl Polym Sci 98:591–597.
Chen J, Zhang W, Li X, Chen Y (2014) Thermal degradation kinetics of a linked polyurethane acrylate film. J Test Eval 42:1–7.
Martins MA, Moreno RM, McMahan CM, Brichta JL, Goncalves PDS, Mattoso LH (2008) Thermooxidative study of raw natural rubber from Brazilian IAC 300 series clones. Thermochim Acta 474:62–66.
Flynn JH, Wall LA (1966) A quick direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B Polym Lett 4:323–328.
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706.
Lu L, Yu H, Wang S, Zhang Y (2009) Thermal degradation behavior of styrene-butadiene-styrene tri-block copolymer/multiwalled carbon nanotubes composites. J Appl Polym Sci 112:524–531.
Budrugeac P (2000) The evaluation of the non-isothermal kinetic parameters of the thermal and thermal-oxidative degradation of polymers and polymeric materials: its use and abuse. Polym Degrad Stab 71:185–187.
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886.
Xu J, Zhang A, Zhou T, Cao X, Xie Z (2007) A study on thermal oxidation mechanism of styrene-butadiene-styrene block copolymer (SBS). Polym Degrad Stab 92:1682–1691.
Pisharath S, Ang HG (2007) Thermal decomposition kinetics of a mixture of energetic polymer and nitramine oxidizer. Thermochim Acta 459:26–33.
Nakazono T, Matsumoto A (2010) Mechanical properties and thermal aging behavior of styrene-butadiene rubbers vulcanized using liquid diene polymers as the plasticizer. J Appl Polym Sci 118:2314–2320.
Cunneen JI (1968) Oxidative aging of natural rubber. Rubber Chem Technol 41:182–208.
Bevilacqua EM, English ES (1961) The scission step in hevea oxidation. J Polym Sci 49:495–505.
Li GY, Koenig JL (2003) FTIR imaging of oxidation of polyisoprene 2. The role of N-phenyl-N′-dimethyl-butyl-p-phenylenediamine antioxidant. Polym Degrad Stab 81:377–385.
Li A (2009) Aging and life prediction of rubber. Rubber Ref 3:2–77.
Hawkins WL (1984) Polymers/properties and applications. Polymer degradation and stabilization, vol 8. Springer, Berlin, pp 45–46.
Acknowledgements
The financial supports from the National Natural Science Foundation of China under Grant No. 51473012 and the Major Research plan from the Ministry of Science and Technology of China under Grant No. 2014BAE14B01 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, W., Jia, Z., Zhang, Z. et al. Improvements of lanthanum complex on the thermal-oxidative stability of natural rubber. J Mater Sci 51, 9043–9056 (2016). https://doi.org/10.1007/s10853-016-0157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0157-4