Abstract
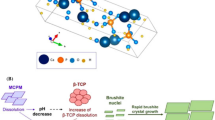
Dicalcium phosphate dihydrate (CaHPO4·2H2O), also known as brushite, is one of the important bioceramics for bone regeneration. However, fast setting of the brushite cement under physiological conditions has limited its clinical use. Furthermore, brushite cement without any additives normally has poor injectability due to the liquid–solid phase separation. In the present study, magnesium-doped β-tricalcium phosphate (Mg-β-TCP) with chemical formula of β-Ca2.96−x Mg x (PO4)2 was used to prepare injectable brushite cements with improved physicochemical properties. β-TCP containing different amounts of Mg2+ ions were reacted with monocalcium phosphate monohydrate [Ca(H2PO4)2·H2O, MCPM] in the presence of water to furnish corresponding brushite cement. The samples were characterized using X-ray diffractometry, Fourier transform infrared spectroscopy and field emission scanning electron microscopy. The effect of magnesium ions on the structural, mechanical, and setting properties of the cements is reported. Our results indicate that the presence of Mg2+ ions increases the degree of injectability, setting time, and mechanical properties of the brushite cement. The compressive strength of brushite cement was substantially increased upon incorporation of Mg2+ ions. Furthermore, the setting times of the brushite cement were significantly improved. Gentamicin sulfate, amoxicillin and ampicillin trihydrate were incorporated into the Mg-brushite cement, and their release profiles showed a sustained drug release over 14 days. Cumulative releases of 99.3, 87, and 79 % were observed for gentamicin sulfate, amoxicillin, and ampicillin trihydrate, respectively.










Similar content being viewed by others
References
Ginebra M-P, Canal C, Espanol M, Pastorino D, Montufar EB (2012) Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev 64:1090. doi:10.1016/j.addr.2012.01.008
Zhang J, Liu W, Schnitzler V, Tancret F, Bouler J-M (2014) Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater 10:1035. doi:10.1016/j.actbio.2013.11.001
Bohner M, Gbureck U, Barralet JE (2005) Technological issues for the development of more efficient calcium phosphate bone cements: a critical assessment. Biomaterials 26:6423. doi:10.1016/j.biomaterials.2005.03.049
Raynaud S, Champion E, Bernache-Assollant D, Thomas P (2002) Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 23:1065. doi:10.1016/S0142-9612(01)00218-6
Han B, Ma P-W, Zhang L-L et al (2009) β-TCP/MCPM-based premixed calcium phosphate cements. Acta Biomater 5:3165. doi:10.1016/j.actbio.2009.04.024
Huan Z, Chang J (2009) Novel bioactive composite bone cements based on the β-tricalcium phosphate–monocalcium phosphate monohydrate composite cement system. Acta Biomater 5:1253. doi:10.1016/j.actbio.2008.10.006
Roy M, DeVoe K, Bandyopadhyay A, Bose S (2012) Mechanical property and in vitro biocompatibility of brushite cement modified by polyethylene glycol. Mater Sci Eng C 32:2145. doi:10.1016/j.msec.2012.05.020
Bohner M, Baroud G (2005) Injectability of calcium phosphate pastes. Biomaterials 26:1553. doi:10.1016/j.biomaterials.2004.05.010
Tamimi F, Sheikh Z, Barralet J (2012) Dicalcium phosphate cements: brushite and monetite. Acta Biomater 8:474. doi:10.1016/j.actbio.2011.08.005
Hofmann MP, Mohammed AR, Perrie Y, Gbureck U, Barralet JE (2009) High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater 5:43. doi:10.1016/j.actbio.2008.08.005
Torres PMC, Gouveia S, Olhero S, Kaushal A, Ferreira JMF (2015) Injectability of calcium phosphate pastes: effects of particle size and state of aggregation of β-tricalcium phosphate powders. Acta Biomater 21:204. doi:10.1016/j.actbio.2015.04.006
Pina S, Torres PMC, Ferreira JMF (2010) Injectability of brushite-forming Mg-substituted and Sr-substituted α-TCP bone cements. J Mater Sci Med 21:431. doi:10.1007/s10856-009-3890-2
Alkhraisat MH, Cabrejos-Azama J, Rodríguez CR, Jerez LB, Cabarcos EL (2013) Magnesium substitution in brushite cements. Mater Sci Eng C 33:475. doi:10.1016/j.msec.2012.09.017
Boanini E, Gazzano M, Bigi A (2010) Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater 6:1882. doi:10.1016/j.actbio.2009.12.041
Lee D, Kumta PN (2010) Chemical synthesis and stabilization of magnesium substituted brushite. Mater Sci Eng C Mater Biol Appl 30:934. doi:10.1016/j.msec.2010.04.007
Cacciotti I, Bianco A (2011) High thermally stable Mg-substituted tricalcium phosphate via precipitation. Ceram Int 37:127. doi:10.1016/j.ceramint.2010.08.023
Xu HHK, Weir MD, Burguera EF, Fraser AM (2006) Injectable and macroporous calcium phosphate cement scaffold. Biomaterials 27:4279. doi:10.1016/j.biomaterials.2006.03.001
Miola M, Bruno M, Maina G, Fucale G, Lucchetta G, Vernè E (2014) Antibiotic-free composite bone cements with antibacterial and bioactive properties. a preliminary study. Mater Sci Eng C Mater Biol Appl 43:65. doi:10.1016/j.msec.2014.06.026
Kyllönen L, D’Este M, Alini M, Eglin D (2015) Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater 11:412. doi:10.1016/j.actbio.2014.09.006
Alkhraisat MH, Rueda C, Cabrejos-Azama J et al. (2010) Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater 6:1522. doi:10.1016/j.actbio.2009.10.043
Canal C, Pastorino D, Mestres G, Schuler P, Ginebra M-P (2013) Relevance of microstructure for the early antibiotic release of fresh and pre-set calcium phosphate cements. Acta Biomater 9:8403. doi:10.1016/j.actbio.2013.05.016
van de Belt H, Neut D, Uges DRA et al (2000) Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 21:1981. doi:10.1016/S0142-9612(00)00082-X
Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33:5967. doi:10.1016/j.biomaterials.2012.05.031
Bohner M, Lemaître J, Merkle HP, Gander B (2000) Control of gentamicin release from a calcium phosphate cement by admixed poly(acrylic acid). J Pharm Sci 89:1262. doi:10.1002/1520-6017(200010)89:10<1262:AID-JPS4>3.0.CO;2-7
Tamimi F, Torres J, Bettini R et al (2008) Doxycycline sustained release from brushite cements for the treatment of periodontal diseases. J Biomed Mater Res A 85:707. doi:10.1002/jbm.a.31610
Schnieders J, Gbureck U, Thull R, Kissel T (2006) Controlled release of gentamicin from calcium phosphate–poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials 27:4239. doi:10.1016/j.biomaterials.2006.03.032
Marchi J, Dantas ACS, Greil P, Bressiani JC, Bressiani AHA, Müller FA (2007) Influence of Mg-substitution on the physicochemical properties of calcium phosphate powders. Mater Res Bull 42:1040. doi:10.1016/j.materresbull.2006.09.015
Montufar EB, Maazouz Y, Ginebra MP (2013) Relevance of the setting reaction to the injectability of tricalcium phosphate pastes. Acta Biomater 9:6188. doi:10.1016/j.actbio.2012.11.028
Forouzandeh A, Hesaraki S, Zamanian A (2014) The releasing behavior and in vitro osteoinductive evaluations of dexamethasone-loaded porous calcium phosphate cements. Ceram Int 40:1081. doi:10.1016/j.ceramint.2013.06.107
Engstrand Unosson J, Persson C, Engqvist H (2015) An evaluation of methods to determine the porosity of calcium phosphate cements. J Biomed Mater Res B 103:62. doi:10.1002/jbm.b.33173
Hesaraki S, Farhangdoust S, Ahmadi K, Nemati R, Khorami M (2012) Phase evaluation and lattice parameters of strontium/magnesium incorporated beta-tricalcium phosphate bioceramics. J Aust Ceram Soc 48:166
Schroeder LW, Dickens B, Brown WE (1977) Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. II. Refinement of Mg-containing β-Ca3(PO4)2. J Sol State Chem 22:253. doi:10.1016/0022-4596(77)90002-0
Yashima M, Sakai A, Kamiyama T, Hoshikawa A (2003) Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J Sol State Chem 175:272. doi:10.1016/S0022-4596(03)00279-2
Araújo JC, Sader MS, Moreira EL, Moraes VCA, LeGeros RZ, Soares GA (2009) Maximum substitution of magnesium for calcium sites in Mg-β-TCP structure determined by X-ray powder diffraction with the rietveld refinement. Mater Chem Phys 118:337. doi:10.1016/j.matchemphys.2009.07.064
Torres PMC, Abrantes JCC, Kaushal A et al (2016) Influence of Mg-doping, calcium pyrophosphate impurities and cooling rate on the allotropic α ↔ β-tricalcium phosphate phase transformations. J Eur Ceram Soc 36:817. doi:10.1016/j.jeurceramsoc.2015.09.037
Lee D, Sfeir C, Kumta PN (2009) Novel in situ synthesis and characterization of nanostructured magnesium substituted β-tricalcium phosphate (β-TCMP). Mater Sci Eng C 29:69. doi:10.1016/j.msec.2008.05.017
Yanli Cai SZ, Yongsheng Wang, Min Qian, Wenjian Weng (2009) Improvement of bioactivity with magnesium and fluorine ions incorporated hydroxyapatite coatings via sol–gel deposition on Ti6Al4V alloys. Thin Solid Film. doi:10.1016/j.tsf.2009.03.071->
Suchanek WL, Byrappa K, Shuk P, Riman RE, Janas VF, TenHuisen KS (2004) Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical–hydrothermal method. Biomaterials 25:4647. doi:10.1016/j.biomaterials.2003.12.008
Ibrahim D, Mostafa A, Korowash S (2011) Chemical characterization of some substituted hydroxyapatites. Chem Cent J 5:1. doi:10.1186/1752-153X-5-74
Acknowledgements
The authors would like to acknowledge the financial assistance via Geran Unviersity Penyelidikan scheme, Vot Q.J130000.2509.06H20 from Universiti Teknologi Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saleh, A.T., Ling, L.S. & Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J Mater Sci 51, 7427–7439 (2016). https://doi.org/10.1007/s10853-016-0017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0017-2